ABOUT ALTASCIENCES
Altasciences transforms the traditional outsourcing paradigm by simplifying and streamlining drug development solutions, whether for a single study or multiple programs, to offer an integrated/synchronized approach to CRO and CDMO services.
FEATURED CONTENT
-
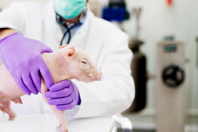
Discover how the Sinclair Nanopig® is advancing preclinical research as a cost-effective, human-relevant alternative to traditional non-rodent models.
-
In this eBook, we answer critical questions and highlight key considerations to help you advance your dermal program from discovery through clinical trials to proof of concept, and beyond.
-
Discover the power of discovery research, key trends shaping early-phase drug development, and two exclusive case studies from our Sacramento facility.
-
Discover how early clinical cognitive testing and dedicated driving simulation studies can help assess the cognitive functions for safety and efficacy in drug development.
-
In this article, we provide a detailed overview of the multiple facets you need to consider during the planning and conduct of your CNS-active drug development.
-
This eBook examines the complex regulatory landscape and the preclinical, clinical, and manufacturing challenges involved in developing Schedule 1 therapeutics.
-
Consider the potential of Sinclair Nanopig™ as a next-generation non-rodent model to enhance drug development, improve regulatory pharmacology, and reduce reliance on traditional non-rodent models.
-
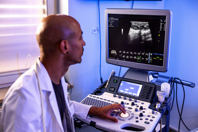
Ultrasound-guided liver biopsies offer a safe, minimally invasive method for repeated tissue collection in non-human primates, ensuring reliable results with minimal animal stress and recovery time.
-
Support the development of standardized SEND guidelines for Animal Rule studies to ensure accurate and consistent regulatory submissions for acute radiation syndrome research.
-
A robust LC-MS/MS assay to quantify CTI-1601 in cynomolgus monkey tissues is essential for understanding its disposition and processing, supporting its potential as a protein replacement therapy.
CONTACT INFORMATION
Altasciences
6605 Merrill Creek Parkway
Everett, WA 98203
UNITED STATES
Contact: contact@altasciences.com
FEATURED SOLUTIONS
-
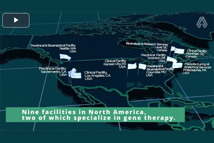
With over 585,000-sq.-ft. of purpose-built space, our preclinical facilities across North America are designed to support your drug development journey, from early discovery to IND/NDA submission.
-
Recognized globally as a CNS Center of Excellence, Altasciences has completed more than 200 preclinical and clinical neurological studies, in addition to providing formulation, manufacturing, and analytical testing services, as well as bioanalytical support. Speak with one of their experts!
-
Altasciences specializes in lead optimization studies, helping you identify the most promising drug candidates through precise, data-driven preclinical screening. Our expert scientists tailor each study to your unique needs, utilizing efficient non-GLP screening protocols to accelerate decision-making. With streamlined study designs, clear data visualization, and rapid turnaround times, we provide actionable insights to advance your drug discovery pipeline.
-
With over 130 in-life gene therapy studies completed, Altasciences is a leading expert in gene therapy research, with integrated preclinical and bioanalytical capabilities.
-
We maintain an unwavering focus on the welfare of the laboratory animals in our care. Our entire preclinical staff is trained in laboratory animal care and focused on animal welfare and environmental enrichment — embracing compassion, sensitivity and adherence to regulatory guidelines.
-
Preclinical immunotoxicology studies play a crucial role in assessing the potential adverse effects of drugs, chemicals, or other substances on the immune system before they advance to first-in-human linical trials.
-
Safety pharmacology is the cornerstone of responsible drug development. It involves the evaluation of a drug candidate's potential impact on vital organ systems such as the cardiovascular, respiratory, and central nervous systems. By identifying any potential safety concerns early in the development process, safety pharmacology studies play a crucial role in minimizing risks to patients and ensuring the overall safety and efficacy of the final product.
-
Leveraging extensive expertise and state-of-the-art facilities, Altasciences conducts comprehensive toxicology evaluations to ensure the safety and regulatory compliance of your novel drug candidates.
-
Leveraging extensive experience with various research lineages of miniature swine, explore our therapeutic and research areas, as well as our applications of the miniature swine model and more.
-
Industry leader in preclinical safety assessments across various therapeutic areas. 30+ years of experience in full range of in vivo non-GLP and GLP studies in both rodent and non-rodent species.