Method For Surgical Closing Of Muscle Biopsy Sites On Non-Human Primates In Group Housing
By David Benedict, Brett Megrath, Christina Cruzen, and DVM DACLAM
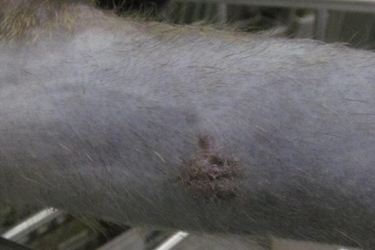
Group housing non-human primates in research settings offers numerous benefits but also presents challenges for study procedures. In a study involving cynomolgus macaques (Macaca fascicularis), multiple muscle biopsy sites were required twice over the course of the study. To improve outcomes, surgical and postoperative care protocols were modified to reinforce surgical closure, enhance analgesia, and prevent postoperative infection while minimizing the duration of single housing. Animals were anesthetized with a combination of ketamine and dexmedetomidine, and pre-operative analgesia was provided using meloxicam. An 8mm biopsy punch was used to make an incision over the muscle, from which two samples per muscle were collected. Both the muscle and skin were closed with a simple interrupted suture pattern (3.0), and surgical staples and adhesive were applied to ensure closure.
Post-surgery, bupivacaine (1 mg/kg) was administered subcutaneously at the incision sites, along with ceftiofur (20 mg/kg) subcutaneously, and atipamezole (0.25 mg/kg) intramuscularly. Additional doses of meloxicam (0.2 mg/kg) were administered for two days after the procedure. Previous procedures had only used sutures and surgical adhesive, with ceftiofur being administered only if postoperative infections were observed. With the introduction of surgical staples and ceftiofur, postoperative complications dropped significantly from around 15% to 2%, and animals were able to return to group housing within 24 hours of surgery. Over the next six days, only brief separations were needed to administer meloxicam and monitor for any complications.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Drug Discovery Online? Subscribe today.
