Intravenous Sampling And Administration In Rodents: Overcoming The Challenges In The Use Of Vascular Access Buttons™ In Rodents
By Gabriela Campoy, Julie Forget, Narine Lalayeva, Adeline Varnet, Serah Tanno, and Norbert Makori
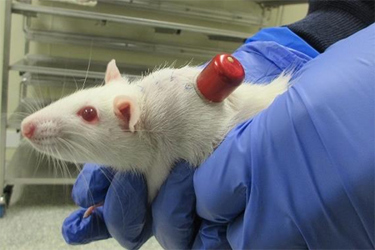
Ensuring reliable and repeated vascular access in rodents for preclinical studies presents a significant challenge, particularly in chronic toxicology models. Vascular access buttons (VABs) provide a permanent transcutaneous catheter system for blood collection and dose administration, reducing stress and enabling group housing. However, a retrospective review of multiple studies highlighted the need for modifications to improve catheter patency and ensure consistent data collection.
In an early study conducted before these modifications, approximately 9% of VAB-implanted animals required veterinary intervention unrelated to the test article, leading to notable histopathological findings and unscheduled euthanasia. To address these issues, subsequent studies incorporated refinements such as an extended acclimation period, routine surgical site cleaning, regular patency checks, more frequent nail trims, and adjustments in husbandry practices, including the use of cellulose bedding and modified food hoppers and enrichment items. These modifications effectively reduced surgical site inflammation and mortality rates.
However, due to smaller sample sizes and limited histopathologic evaluations in follow-up studies, key findings such as injection site inflammation and thrombi could not be thoroughly assessed. Larger-scale studies are necessary to validate these refinements, conduct comprehensive pathological evaluations, and establish reference ranges for clinical pathology and histopathology in surgically implanted rodents, ultimately enhancing the reliability and applicability of VABs in preclinical research.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Drug Discovery Online? Subscribe today.
