Zycos begins Phase II trial HPV- cervical dysplasia
What's significant about this study is that just 18 months ago ZYC101a was a discovery-stage compound. According to Zycos this abbreviated concept-to-clinic time period is an industry record. Zycos believes that, due to the "plug-and-play" nature of its discovery platform, future drug candidates may reach the clinic even faster because preclinical safety tests might not be needed.
ZYC101a, which works with the body's own defense system, contains a piece of genetic code that is naturally processed by the immune system and is subsequently used to signal disease-fighting T cells to attack cervical cells infected by HPV.
Pre-cancerous cervical infections are commonly referred to as cervical dysplasia. In the United States, between one and two million are diagnosed every year with the disease, which if left untreated can progress to cancer. Treatment options today involve invasive procedures that permanently remove cervical tissue and can lead to childbearing complications. HPV is also associated with cervical cancer, anal dysplasia, anal cancer, genital warts, and some head and neck cancers. A Phase I/II study for ZYC101a in anal dysplasia patients is currently ongoing at the University of California, San Francisco.
Core technology safes development time
Zycos's claim to fame is a plasmid DNA carrier dubbed Biotope, which expresses therapeutic peptides or proteins. Once inside the cell, Biotope-encoded information is expressed through natural cellular processes. When the peptides are antigens, they get presented on the cell surface, which then activates the immune system.
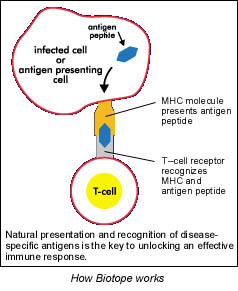
The regulatory benefit of all this is that plasmid DNA is a non-viral material that is considered to be a "well-characterized" biologic by the FDA. Most experts agree that plasmid DNA is non-integrating and non-replicating, therefore non-pathogenic.
Zycos uses the Biotope to create a pipeline of drugs by formulating multiple Biotope plasmid DNA constructs, each with a different set of disease-specific information. The result is a "plug-and-play" series of potential therapeutics whose safety is already well-characterized.

To augment Biotope, Zycos has developed Ensphere, a delivery system that protects and delivers biological drugs, including plasmid DNA, to cells in the body with minimal degradation. Ensphere is polymer-based technology, which when combined with Biotope produces plasmid DNA vaccines encapsulated in microspheres. DNA administered with Ensphere requires 100-fold less DNA to achieve a similar level of immune response as naked DNA injections.
For more information, contact Melissa Chen of Zycos at 781-274-6500, ext. 211.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online and Pharmaceutical Online
adepalma@vertical.net
