Valentis and Viragen will co- develop PEGylated alpha interferon for hepatitis C
Believed to be more stable and effective with fewer side effects
On September 19, Valentis (Burlingame, CA) announced that its subsidiary, PolyMASC Pharmaceuticals plc, will collaborate with Viragen Inc. (Plantation, FL) on a new treatment for hepatitis C.
The collaborators will use PolyMASC's novel protoMASC PEGylation process to deliver Viragen's lead drug, Omniferon, a natural alpha interferon currently in Phase II clinical trials in Europe for hepatitis C. Financial terms of the collaboration were not disclosed.
Viragen chose the PolyMASC PEG (polyethylene glycol) adduct system for its ability to preserve interferon's activity while protecting it from degradation in the bloodstream. Those benefits translate to higher efficacy and lower required dosages. Since Omniferon is a natural form of alpha-interferon, both companies believe it will show an improved side-effect profile compared with recombinant products.
PolyMASC creates improved versions of biopharmaceuticals by attachment of PEG without linkers under very mild, almost physiologic conditions. Depending on the protein, that can reap tremendous benefits by preserving nearly all the original protein's biological activity.
PolyMASC's PEGylation process relies on nucleophilic substitution and therefore uses no linking agent. Proteins or peptides are linked directly to the PEG modifier.

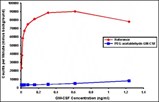
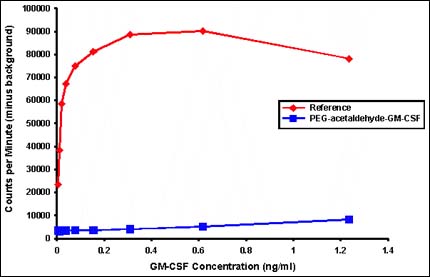
How PEG is added to proteins can make all the difference. Here, GM-CSF PEGylated through the traditional PEG-acetaldehyde method loses almost all its activity (blue line, bottom) compared with the native protein (red line, top). PolyMASC's PEG adducts do not inactivate therapeutic proteins.
PEGylation also improves a drug's staying power. Many small peptides eliminated through the kidneys persist longer in the body when joined with PEG. Drug loss at the injection site can also be extremely high. In these cases, a patient's treatment could require multiple injections to achieve the desired dosage. Inefficient drug delivery systems subject patients to additional injections and drug-related side effects since a significant percentage of the drug is essentially wasted.
For more information: Mel Rothberg, Executive Vice President, Viragen, Inc., 865 SW 78th Avenue, Suite 100, Plantation, FL 33324. Tel: 954-233-8746.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online
