Tripos and BMS agree to design integrated research informatics system
Collaboration aims at accelerating drug discovery
On December 19, Tripos Inc. (St. Louis) announced that it had begun the first phase of a program with Bristol-Myers Squibb Co. (BMS; Princeton, NJ) and Andersen Consulting (Chicago) to design and implement a new, integrated research informatics system. Tripos will work with Bristol-Myers Squibb to develop a new decision support capability to accelerate drug discovery. Financial terms were not disclosed.
Tripos will provide software design and implementation services to build a global desktop system to facilitate analysis of information throughout BMS. The system will incorporate Tripos' proprietary MetaLayer technology, a powerful combination of middleware and application development frameworks. Bristol-Myers Squibb researchers and managers worldwide will use the system as their primary drug discovery information portal, improving access to a wider range of scientific data and information, including easy access to calculated methods and the ability to analyze and compare calculated results with experiments.
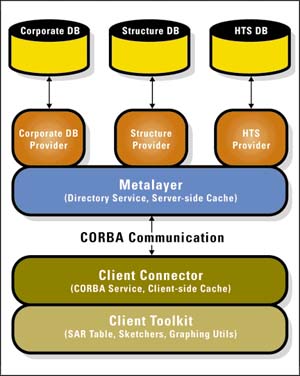
The Tripos MetaLayer platform provides a single portal to diverse scientific research information.
"The pharmaceutical industry is facing an unprecedented urgency to develop informatics systems that help access and analyze vast amounts of data from diverse sources," said John P. McAlister, president and CEO of Tripos. "We are excited about building on our relationship with Bristol-Myers Squibb to help create an integrated global portal to manage, analyze and visualize a wide range of discovery research data."
MetaLayer technology provides a single desktop portal to dispersed, heterogeneous scientific research information from gene sequence to qualified drug lead candidate. Using MetaLayer, users can store, retrieve, coordinate, manage, display, analyze, and transfer biological, chemical, and molecular structure data to different applications. As a result, they are better able to manage, optimize and analyze their genomic, high-throughput screening, chemical, and related pre-clinical scientific information.
"In drug discovery today, advanced information systems are needed to manage the knowledge required to make effective decisions," said Paul Weber, vice president of Software Consulting Services for Tripos. "Tripos' MetaLayer is becoming the standard in the pharmaceutical industry for integrating diverse data sources, and for providing a rich desktop Java application development environment."
For more information, contact Colleen Martin, chief financial officer of Tripos, at 314-647-1099 or colleen@tripos.com.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online and Pharmaceutical Online
adepalma@vertical.net
