The Evolving Landscape Of Drug Discovery Services
By Dipak Mahajan, MarketsandMarkets

Pharma and biotech companies are set to pursue partnerships with drug discovery service providers at an increased rate in the years ahead. And it’s not just small and emerging drug companies; it’s also Big Pharma companies such as Pfizer, Novartis, and Amgen.
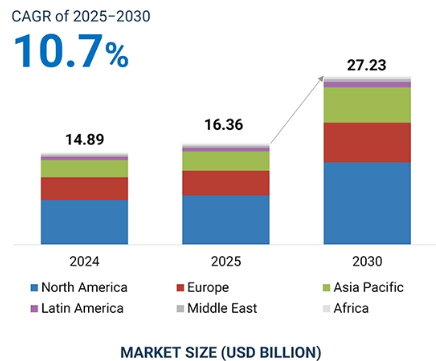
The global drug discovery services market is projected to reach $27,229.3 million by 2030 from $16,364.0 million in 2025, at a CAGR of 10.7% during the forecast period. Market growth is primarily driven by the growing R&D expenditure in the pharma and biopharma industry, the need for outsourcing processes such as analytical testing due to an increasing R&D pipeline, growing research into rare diseases and orphan drugs, and the high cost of in-house drug development.
Technological advancements and new drug discovery techniques, the expiry of patents, and rising demand for specialized testing services are also expected to offer a wide range of growth opportunities in this sector. Meanwhile, reduced R&D budgets in the pharmaceutical sector and economic uncertainties due to the global landscape are limiters to what could be more rapid growth.
Many interconnected stakeholders play an important role in each new drug discovery process. Academic institutions conduct early-phase research to aid drug companies, while CROs offer different drug discovery services, including processes such as identifying screening hits, medicinal chemistry, and the optimization of hit cells.
The drug discovery services market has experienced significant trends and disruptions that have reshaped the landscape. The integration of artificial intelligence and machine learning is simplifying the approach to drug discovery. The continued growth in outsourcing of research services enables pharma and biotech companies to shift their focus onto their core competencies and utilize external expertise. In addition, increasing demand for personalized medicine is influencing service providers to enhance their offerings with more targeted and patient-specific approaches. The growth of CROs and specialized biotech companies has led to a more fragmented and competitive landscape where companies are pressured to differentiate and innovate their services. With global regulations growing stricter, the need for compliance and data security is also increasing, so there is a higher impact on the way companies cooperate with service providers. These changes increase the pressure for businesses to compete in many different aspects, which requires innovation flexibility, advanced systems, and various other factors while managing operational costs effectively.
Next, we’ll discuss the most popular core technologies (which are directly applied in drug discovery processes), complementary technologies (which provide additional capabilities or efficiencies that augment core drug discovery workflows), and emerging technologies (innovations not yet core to drug discovery workflows but could enhance the process in the future), and we’ll also cover other trends.
3 Most Popular Outsourced Core Technologies in Drug Discovery
High-Throughput Screening
High-throughput screening (HTS) is a key technology in drug discovery, enabling the rapid assessment of extensive chemical libraries to identify compounds that interact with specific biological targets. Utilizing automation, miniaturized assays, and advanced data processing, HTS facilitates the efficient concurrent evaluation of numerous samples. This approach has become integral to pharmaceutical and academic research, expediting the identification of potential therapeutic agents. The process involves preparing assay plates, typically high-density microtiter plates, where each well contains a distinct compound and the biological target of interest. Robotic systems manage the precise dispensing of reagents and samples, ensuring consistency and high throughput. Detection methods, often fluorescence or luminescence-based, measure the biological responses, generating substantial data for analysis. Advancements in HTS technologies have led to the adoption of ultra-high-throughput screening (HTS), which can evaluate over 100,000 compounds daily. Despite its efficiency, HTS faces challenges such as data management complexities and the potential for false positives or negatives, necessitating subsequent validation processes. Nonetheless, HTS remains a cornerstone in the early stages of drug development, significantly reducing the time and cost of bringing new therapeutics to market.
CRISPR Gene Editing
CRISPR gene editing has become a pivotal tool in drug discovery, enabling precise modifications of genetic material to facilitate the development of targeted therapies. This technology allows for the accurate editing of genes associated with various diseases, thereby aiding in identifying and validating potential drug targets. In the context of monogenic disorders, CRISPR has been utilized to correct specific genetic mutations, offering potential cures for conditions such as sickle cell anemia and B-thalassemia. Beyond genetic disorders, CRISPR has been applied in oncology to modify immune cells, enhancing their ability to recognize and attack cancer cells. This approach includes engineering T cells to express CAR T cells, which have shown efficacy in treating certain hematologic malignancies.
CRISPR-based high-throughput screening enables systematic investigation of gene function, facilitating the discovery of novel drug targets and the elucidation of drug resistance mechanisms. The versatility of CRISPR technology extends to the development of disease models, including the generation of genetically modified animals and organoids that closely mimic human pathophysiology, thereby providing robust platforms for preclinical drug testing. Overall, CRISPR gene editing is a transformative tool in drug discovery, accelerating the development of precision medicines across a spectrum of diseases.
Structure-Based Drug Design/Computer-Aided Drug Design
Structure-based drug design (SBDD) and computer-aided drug design (CADD) are integral methodologies in modern drug discovery, utilizing computational tools to enhance the efficiency and precision of developing therapeutic agents. SBDD involves the use of detailed structural information about biological targets, such as proteins, to guide the design and optimization of molecules that can interact specifically with these targets. This approach often employs molecular docking techniques, which predict the preferred orientation of a molecule when bound to a target, facilitating the identification of compounds with potential inhibitory effects.
The process includes iterative cycles of computational modeling and experimental validation to refine the interactions between the drug candidate and its target. CADD encompasses a broader spectrum of computational strategies, including both structure-based and ligand-based approaches. These methods integrate data from various sources to model and predict the behavior of drug candidates, thereby streamlining the discovery process. The integration of machine learning and artificial intelligence within CADD has further enhanced its predictive capabilities, enabling the analysis of complex biological data and the identification of novel therapeutic compounds. As these technologies advance, they continue to play a pivotal role in rationalizing and expediting the development of new drugs.
3 Most Popular Outsourced Complementary Technologies In Drug Discovery
Automated Liquid Handling
Automated liquid handling systems are integral to modern drug discovery processes, enhancing efficiency and precision in laboratory workflows. These systems use robotic mechanisms to accurately dispense and manage liquid samples, thereby minimizing manual intervention and reducing the potential for human error. In HTS, automated liquid handlers facilitate the rapid testing of extensive chemical libraries against biological targets by enabling precise and reproducible dispensing of reagents into microplates. This automation accelerates the identification of active compounds and streamlines subsequent validation processes.
In next-generation sequencing (NGS) applications, automated liquid handling ensures consistent sample preparation, which is critical for obtaining reliable sequencing data. The integration of these systems in drug discovery laboratories not only improves throughput but also enhances data quality by ensuring uniformity in sample handling. As a result, automated liquid handling has become a cornerstone technology, complementing other advanced methodologies to expedite the development of new therapeutic agents.
In Silico Modeling
In silico modeling has become essential in drug discovery, using computational techniques to simulate and analyze biological and chemical systems. This approach encompasses various methodologies, including structure-based and ligand-based modeling, to predict the interaction between potential drug candidates and their biological targets. Structure-based modeling involves the use of three-dimensional structures of biological targets, obtained through techniques like X-ray crystallography or nuclear magnetic resonance (NMR) spectroscopy, to design molecules that fit precisely into the targets active site.
Patient-Derived Xenografts And Organoids
Patient-derived xenografts (PDX) and organoids are pivotal technologies in drug discovery, offering advanced platforms for preclinical evaluation of therapeutic agents. PDX models involve implanting human tumor tissues into immunodeficient mice, thereby preserving the histological architecture and genetic profile of the original patient tumor. This approach enables researchers to study tumor behavior and drug responses in a living organism, closely mimicking human cancer progression. PDX models have been instrumental in assessing the efficacy of novel drugs, screening for patient-specific drug sensitivities, and understanding mechanisms of drug resistance. For instance, studies have demonstrated that PDX models can accurately predict clinical outcomes and guide personalized treatment strategies.
Organoids are three-dimensional structures cultured from patient-derived cells that replicate the complex architecture and functionality of human organs or tumors in vitro. These miniaturized models maintain the genetic and phenotypic characteristics of the source tissue, making them valuable for high-throughput drug screening and disease modeling. Organoids have been utilized to evaluate drug responses in various cancers, facilitating the identification of effective therapies tailored to individual patients. Moreover, organoid cultures allow for exploring tumor microenvironment interactions and testing combination therapies in a controlled setting. The integration of organoid technology into drug discovery pipelines enhances the predictive accuracy of preclinical studies and accelerates the development of personalized medicine approaches. Combining PDX models and organoid systems offers a comprehensive platform for translational research, bridging the gap between in vitro studies and clinical applications. This integrated approach enables the validation of therapeutic targets and the optimization of treatment regimens, ultimately contributing to more effective and individualized cancer therapies.
3 Most Popular Outsourced Emerging Technologies in Drug Discovery
Nanotechnology
Nanotechnology has become a significant component in drug discovery, offering innovative solutions for drug development and delivery. By manipulating materials at the nanoscale, researchers can design systems that enhance the solubility, stability, and bioavailability of therapeutic agents. Nanoparticles, such as liposomes, dendrimers, and metallic nanoparticles, serve as drug carriers, enabling controlled and targeted delivery to specific tissues or cells. This targeted approach aims to increase therapeutic efficacy while minimizing side effects. For instance, liposomal formulations have been utilized to encapsulate chemotherapeutic agents, improving their delivery to tumor sites and reducing systemic toxicity. In addition to drug delivery, nanotechnology contributes to drug discovery by facilitating the development of diagnostic tools and imaging agents. Nanoparticles can be engineered to bind to specific biomarkers, allowing for the detection and monitoring of diseases at early stages. This capability is particularly valuable in oncology, where early detection is crucial for effective treatment. Moreover, nanotechnology enables the creation of nano sensors that can detect biological signals, providing real-time data on disease progression and treatment response. Nanotechnology aids in overcoming challenges associated with conventional drug delivery systems, such as poor water solubility and rapid clearance from the body. By designing nanoparticles with specific surface properties and sizes, researchers can enhance the circulation time of drugs and improve their accumulation at target sites through passive targeting mechanisms like the enhanced permeability and retention (EPR) effect. This approach is particularly beneficial in treating diseases such as cancer, where achieving high local drug concentrations is essential for therapeutic success. Nanotechnology serves as an adjacent technology in drug discovery by providing advanced platforms for drug delivery and diagnostics and overcoming the limitations of traditional drug formulations. Its integration into the drug development pipeline has the potential to accelerate the discovery of new therapeutics and improve patient outcomes.
Synthetic Biology
Synthetic biology involves designing and constructing novel biological parts, technologies, and systems and re-engineering current biological systems for pragmatic uses. In drug development, it helps to create new medicinal molecules and better drug delivery methods. Synthetic biology has made it possible to mass produce medications such as artemisinin, a malaria medicine, by engineering microorganisms to create complicated natural chemicals. Synthetic biology helps in medication development by building synthetic gene circuits that can identify illness signs and react to generate medicinal molecules. This method makes it possible to create living therapeutics capable of adjusting to the surroundings and offering continuous treatment. Synthetic biology helps to overcome obstacles related to traditional drug delivery systems, such as low water solubility and fast bodily clearance. Through passive targeting processes, including the EPR effect, researchers can increase the circulation duration of medications and optimize their accumulation at target areas by developing nanoparticles with surface features and sizes. Synthetic biology provides enhanced platforms for drug transport and diagnostics and overcomes the constraints of conventional drug formulations, so it acts as an adjacent technology in drug research. Its inclusion into the medication development process could hasten the identification of new treatments and enhance patient outcomes using the acceleration of discovery.
Organ-On-A-Chip Technology
Organ-on-a-chip (OOC) technology involves creating microfluidic devices that replicate the structure and function of human organs. These devices consist of microchannels lined with living human cells, allowing researchers to study organ-level physiology and disease processes in vitro. In drug discovery, OOC models provide a platform for evaluating drug efficacy and toxicity, potentially reducing reliance on animal testing. For example, Merck researchers are developing liver and intestine OOC models to improve in vitro safety testing of experimental drugs. OOC systems can simulate complex organ interactions, offering insights into multi-organ responses to drugs. This capability is particularly valuable for assessing drug metabolism and systemic effects. The integration of multiple organ models on a single chip enables the study of pharmacokinetics and pharmacodynamics in a controlled environment. Additionally, OOC platforms can be personalized using patient-derived cells, facilitating precision medicine approaches in drug development. Despite their potential, challenges remain in standardizing OOC models and ensuring their predictive accuracy. Ongoing research aims to address these issues and integrate OOC technology into routine pharmacological and medical applications.
Drug Discovery Services Are Being Used The Most For These Indications
In 2024, the oncology segment accounted for 27.9% of the drug discovery services market. The large share of this segment can be attributed to the high incidence of cancer, the growing number of cancer therapeutics, and rising drug development efforts. The market for infectious diseases is projected to reach USD 738.2 million by 2030 from USD 418.7 million in 2025, at the highest CAGR of 12.0% during the forecast period.
The neurology segment will reach USD 3,056.4 million by 2030 from USD 1,852.5 million in 2025, at a CAGR of 10.5% during the forecast period.
The cardiovascular disease segment accounted for 5.2% of the global drug discovery services market in 2024, primarily due to the high prevalence of cardiovascular diseases, the rising geriatric population, and the increasing partnerships between CROs and pharmaceutical & biotechnology companies.
Recent Developments
- In February 2025, Variational Al (Canada) completed the oversubscribed $5.5 million seed extension round. Enki (platform of Variational Al) empowers biopharmaceutical chemistry teams to efficiently discover and optimize novel hits and leads, accelerating early-stage drug discovery.
- In January 2025, The University of Chicago and healthcare investment firm Deerfield Management (U.S.) announced the launch of Hyde Park Discovery, a collaboration aiming to advance the development of new medicines and other lifesaving treatments for disease. Over the next 10 years, Deerfield will provide up to $130 million in targeted funding and operational and scientific expertise to advance The University of Chicago’s discoveries with the potential to become novel therapies.
- In January 2025, Charles River Laboratories announced a partnership with NJ Bio, Inc., a CRO focused on integrated chemistry and bioconjugation services, toward assisting clients in advancing antibody-drug conjugate (ADC) development from concept to clinic.
Regional Trends: Growth Of the Use of Drug Discovery Services
Key Service Providers in Drug Discovery
- Thermo Fisher Scientific Inc. (U.S.)
- Labcorp (U.S.)
- Eurofins Scientific (Germany)
- Charles River Laboratories (U.S.)
- WuXi AppTec (China)
- Pharmaron (China)
- Evotec (Germany)
- Syngene International Limited (India)
- Curia Global, Inc. (U.S.)
- Aragen Life Sciences Ltd. (India)
- Jubilant Pharmova Limited (India)
- Dr. Reddy’s Laboratories Ltd. (India)
- Frontage Holdings Corporation (U.S.)
- GenScript (U.S.)
- Sygnature Discovery (U.K.)
- Piramal Enterprises Ltd. (India)
