2024 Survey Findings: How Is The "Lab Of The Future" Becoming Reality?
By Becky Upton, Ph.D., president, The Pistoia Alliance

In line with our technology focus, the Pistoia Alliance conducted its first Lab of the Future survey in 2023 with Open Pharma Research, as a pulse check on which technologies the industry is investing in and its progress integrating new tech into the lab. Following the success of the report, this year we repeated the survey among 200 life sciences R&D experts spanning Europe, the Americas, and APAC. The goal of the research remains the same: What does the industry need to make the tech of tomorrow a reality in the labs of today?
AI's Growing Role In R&D
It wouldn’t be a 2024 survey without a focus on AI, which 68% of respondents now say they are using in their laboratory work. This finding is up by 14% compared to last year. AI’s momentum is likely to continue, with the survey finding AI and machine learning (ML) are cited as the top tech investments organizations plan to make over the next two years (62%). In comparison, companies are focusing less on infrastructure investments such as cloud platforms (37%), electronic lab notebooks (ELNs) (23%), and laboratory information management systems (LIMS) (20%) (refer to Figure 1). This is because many labs already have some form of these technologies in place, so the focus has now shifted onto other technologies such as AI. Investment in people is also higher in 2024 compared to 2023, as companies seek to hire experts who can help them get the most out of their technology investments, and upskill current staff.
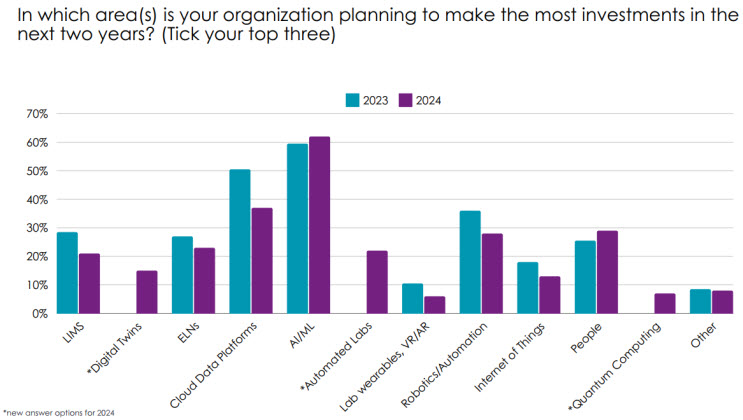
Figure 1
There are some notable AI use cases the industry is getting excited about. For example, AI is significantly accelerating existing workflows in small molecule discovery and lead optimization for new drug candidates – shaving months off time-intensive processes. AI-driven non-animal models have the potential to streamline drug testing. Regulatory bodies are increasingly supportive of the long-term replacement of animal models, with new legislation such as the FDA Modernization Act 2.0. Elsewhere, in the clinical space, AI is supporting precision medicine and better decision-making by analyzing genetic and large data sets of real-world patient data.
However, with all use cases the industry must note that AI will complement, not replace, existing methods and human researchers. AI will indeed become a powerful tool in the life sciences and pharma industries, but it won’t be a panacea. While AI accelerates drug discovery, optimizes development, and reduces costs, it will be one of many tools used in R&D and healthcare.
Three Major Challenges: Privacy, Security, Compliance
The rapid adoption of AI has brought fresh challenges, with respondents citing issues around privacy, security, and regulatory compliance (Figure 2) as potential barriers to implementation.
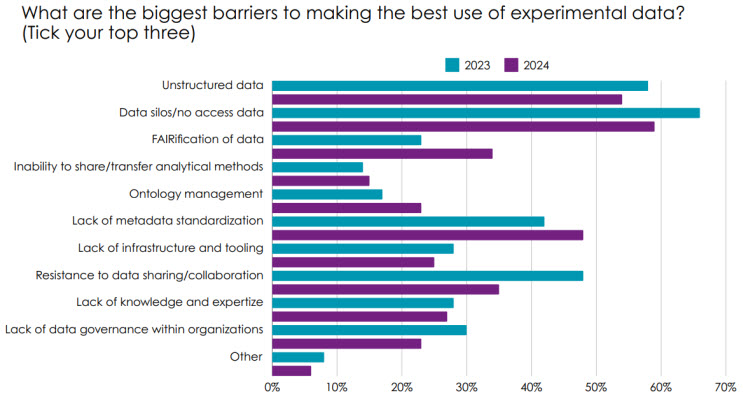
Figure 2
One likely contributor to these concerns is the rise of new AI legislation. The EU AI Act introduced in 2024 will be one of the world’s most stringent; the regulation is particularly relevant to pharma due to the bloc’s large market size and its tendency to set standards that other regions follow. The EU’s rules are based on AI’s potential risk and level of impact to consumers and their data. High-risk applications such as medical devices, drug manufacturing, and diagnostic AI will require conformity assessments, while limited-risk applications such as chatbots must be clearly labeled as AI tools.
Such new regulations are necessary to shape an ethical AI future, but the lack of harmonization and clarity on compliance creates uncertainty for companies – especially those operating across multiple jurisdictions as many large pharma, biotech, and research organizations do. AI is new territory for both legislators and companies that must be navigated together. Trust in AI will only grow with coordinated efforts to ensure data security and ethical practices are embedded.
Foundational Data Challenges Persist
Underpinning the successful use of any new technology, including AI and machine learning, is putting in place a strong data backbone. One change since the 2023 survey is observed in the barriers to utilizing experimental data. Last year, cultural resistance to data sharing was cited by 48% of respondents, but in 2024 that’s down to 35%. This suggests companies are more open to pre-competitive collaboration, as organizations increasingly realize the value of sharing expertise on areas like best practices for adopting new technologies — which has a huge cost and time benefit.
While there is a desire and openness toward collaborating externally, there are still silos of data within organizations. Many organizations are struggling with content-related challenges, such as the ability to access data (cited by 59%), unstructured data (54%), and lack of metadata standardization (48%). Having information that is isolated across departments prevents efficient collaboration and insights. Data integration is another obstacle, as different systems and formats make it difficult to aggregate and analyze diverse data sources effectively. Finally, scalability and storage of growing data volumes require robust systems to ensure accessibility without escalating costs.
Practical Steps For Future Labs
The communal recognition of these challenges emphasizes the opportunity for the life sciences industry to collaborate and overcome these obstacles. Sharing knowledge – and risk – is critical for the industry to collectively reap the benefits of lab of the future technologies and deliver new therapies to patients.
Some of the resources and actions our survey respondents call for include:
- More data governance frameworks (49%)
- Best practice guides and “how-to’s” (45%)
- Help with sophisticated data science and data management techniques – in particular, 51% want more maintenance and management of data standards and ontologies
- Training opportunities – 29% of respondents are seeking ontologies training (up from 18% in 2023) and 32% are looking for education on FAIR data
Better Together
As generative AI and its associated legislation evolve rapidly, the need for collective action in addressing these challenges has become more pressing. As we move into 2025, the industry focus must shift from merely adopting technologies such as AI to establishing the frameworks necessary for their success. Leadership plays a pivotal role here; effective AI use requires a commitment to strong data governance and an organizational culture that promotes cross-functional collaboration and investment in advanced infrastructure. Further, collaboration with regulators is just as vital as internal innovation to ensure compliance and ethical use of AI.
The Pistoia Alliance network has more than 200 members, including pharma, healthcare providers, academic institutions, government bodies, and not-for-profit/B-corps. From our new ontology training to our established FAIR data and AI communities, our mission is to continue to be the go-to organization where companies collaborate to deliver the benefits of data and technology across the global life science community.
 About The Author:
About The Author:
Becky Upton, Ph.D., is president of The Pistoia Alliance.
