Size Matters: The Sinclair Nanopig® As A Translational Non-Rodent Model In Preclinical Research
Drug developers and scientists continually seek animal models that are cost-effective, scientifically robust, and aligned with research objectives while minimizing test article (TA) usage, maintaining data quality, and regulatory compliance. Within the continuum of preclinical development, selecting the right species is critical for generating the safety and toxicology data needed to advance a novel therapeutic into clinical trials.
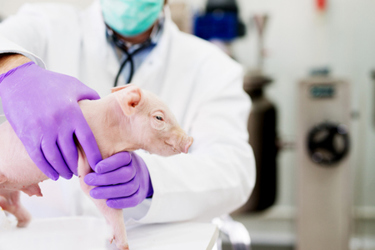
Among non-rodent models, minipigs — and particularly the smaller Sinclair Nanopig® — have gained prominence for their remarkable anatomical and physiological similarity to humans. Their size, metabolism, and organ structure make them highly relevant for diverse therapeutic areas while offering practical and economic advantages over traditional models.
In this issue, we explore how the Sinclair Nanopig® delivers scientific rigor and cost efficiency in preclinical programs, and we highlight a case study demonstrating its advantages over canine models, including reduced incidence of emesis and improved study outcomes.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Drug Discovery Online? Subscribe today.
