Restoring Immune Homeostasis As A Development Approach To Treat And Modify Inflammatory Disease
By Martin Dahl, Ph.D., senior vice president, research, AnaptysBio, Inc.

The intensity of our immune system’s response to potential foreign and internal threats is regulated by an intricate network of checks and balances. In a homeostatic, or normal, state, the immune system recognizes and attacks pathogens, such as viruses or bacteria, while remaining non-reactive to self-antigens that keep us healthy.
However, in a dysregulated or overactive state, the immune system is unable to tune down runaway inflammatory responses to foreign threats and may react against self-antigens in a self-perpetuating cycle that results in dysregulated immune cell activity, immune cell proliferation, and secretion of inflammatory cytokines. This is what happens in inflammatory diseases such as atopic dermatitis (AD), rheumatoid arthritis (RA), and ulcerative colitis (UC).
Co-inhibitory receptors, such as BTLA (B and T lymphocyte attenuator) and PD-1 (programmed cell death-1), are found on the surface of immune cells, such as T cells, B cells, and dendritic cells. They are key to regulating natural inflammatory responses, turning it up when necessary and down when the need has passed, helping to maintain immune homeostasis.1 Therapeutically targeting and activating these co-inhibitory receptors offers an avenue to modulate the immune system’s activity.1
Several companies, including Anaptys, are developing specific antibodies — called immune cell modulators — that in the setting of autoimmune disease, agonize immune cells and return the immune system to a more homeostatic state and potentially modify disease.
Potential of Agonizing Cells to Regulate Inflammatory Disease
Nearly 30 years ago, the role of immune co-inhibitory receptors came into focus after the discovery of the role of PD-1 in regulating the immune system by Tasuku Honjo, Ph.D., which earned him a Nobel Prize.2 This research led to the development of PD-1 antagonist “immuno-oncology” therapies that intentionally hyperactivate the immune system to better detect and destroy cancer cells3 — the opposite of a “homeostatic state.”
This supported a deeper mechanistic understanding of these pathways and led to the discovery that therapeutically agonizing these co-inhibitory receptors can engage the endogenous regulators that are disrupted in the context of systemic and heterogenous autoimmune and inflammatory diseases. For example, targeting PD-1, which is preferentially found on activated T cells, with agonist monoclonal antibodies (mAbs) has the potential to tamper down (and importantly, not fully suppress) an out-of-control inflammatory system in the setting of disease. While this has been challenging research,4,5 there are now a number of co-inhibitory agonists in development evaluating this approach to potentially restore immune balance in patients living with autoimmune and inflammatory diseases.4
This novel, potential game-changing approach may broadly impact the severity and progression of diseases such as AD, RA, and UC. Thus far, the PD-1 agonist class has shown clinical efficacy from a Phase 2a clinical study in RA with compelling proof-of-mechanism, no serious treatment-related adverse events, and efficacy results sustained through six months.6
There are many ongoing proof-of-concept clinical trials across autoimmune diseases which will further our understanding of how to optimize the benefit of agonist mAbs without increasing potential risks of infections or other conditions, while returning the immune system back to a state of homeostasis.6
Here, we discuss three considerations when developing immune cell modulators.
1. Have A Deep Understanding of Immunology
Immunology is an evolving science, and the development of any antibody targeting a co-inhibitory receptor on an immune cell should start from a thorough understanding of the target and its role in a normal immune response as well as how it changes in the diseased state.
Previous research has shown that PD-1 naturally prevents autoimmunity by inhibiting the activity of autoreactive lymphocytes.7 To enhance or optimize the immune effects regulated by PD-1, it’s essential to understand how PD-1 interacts with its respective ligand(s) on opposing immune cells, and what is contributing to the activation or inhibition of an immune response. This knowledge is gained by cultivating a deep understanding of immune cells, their signaling mechanisms, and their interconnections to broader immunological responses, observed in the laboratory and clinical development.
For example, at Anaptys, we’re focused on developing a best-in-class antibody targeting PD-1 with the potential to treat various autoimmune and inflammatory diseases driven by abnormal, dysregulated, or “runaway” inflammation where PD-1 expression, specifically on activated immune cells, is elevated. We have spent over a decade immersed in the science of engaging PD-1, using the latest structural and mechanistic approaches to ensure our novel antibodies are engineered to induce the biologic activity needed to maximize therapeutic effect. Our PD-1 agonist antibody, rosnilimab, is optimized to both deplete those expressing high levels of PD-1 (“PD-1high”) T cells and agonize PD-1intermediate-expressing T cells, which together will contribute to potent immunological outcomes to tackle underlying autoimmunity and inflammation.8
Importantly, we and others have shown that targeting mAbs to the membrane-proximal extracellular regions of co-inhibitory receptors, while not blocking the natural ligand, represents one key component of engaging the endogenous signaling pathways, thereby producing downstream agonistic effects, such as reducing T cell activity, which contributes to the overall reduction of immune activation.7
To potentiate PD-1 agonism, agonist antibodies also need to engage an Fc receptor through the backbone of the antibody called the Fc domain, that enhances PD-1 crosslinking.9 Membrane-proximal binding and crosslinking across two immune cells via Fc receptor engagement contributes to cell-to-cell communication, enabling tight immune synapse formation (Figure 1).8,9
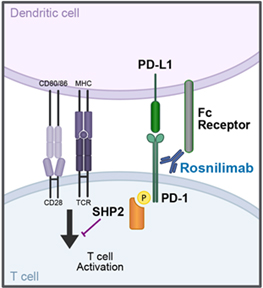
Figure 1. Proposed rosnilimab mechanism of action.
These PD-1 binding properties and Fc receptor binding characteristics contribute to optimal agonism of PD-1, resulting in the inhibition of T cell signaling when the opposing cell is an antigen presenting cell, and potent depletion of PD-1high-expressing T cells when the opposing cell is capable of mediating cell killing (Figure 2).8
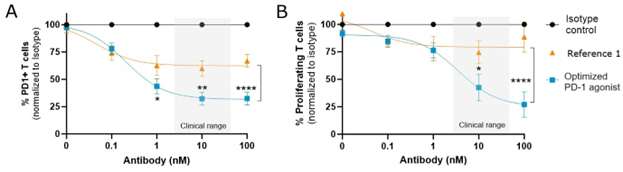
Figure 2. A. Preclinical data showing depletion of PD-1+ T cells by a membrane-proximal binding optimized PD-1 agonist is more potent than the membrane-distal binding reference B. Agonism by a membrane-proximal binding optimized PD-1 agonist more potently reduces T cell proliferation than the membrane-distal binding reference 1.
2. Have a Comprehensive Knowledge of Disease Biology
Similarly, we believe a comprehensive understanding of disease biology is crucial to fully comprehend how the immune system will respond in vivo.
In autoimmune and inflammatory diseases, PD-1 expressing T cells, and most prolifically PD-1high expressing T cells, are hypothesized to be responsible for secretion of inflammatory cytokines leading to tissue damage and perpetuation of the inflammatory cycle. In healthy individuals, a very low level of T cells is PD-1high and less than 15% of T cells are PD-1 expressing.10 These are baseline levels to which we aim to return patients with autoimmune and inflammatory diseases to achieve homeostasis.
For example, in RA, immune dysfunction is observed through the high number of T cells that infiltrate and remain active in the synovium of a joint, where approximately 80% of the infiltrating T cells express PD-1. Increases in circulating T cells in the blood also can be observed, with a 1.5-fold increase of T cells expressing PD-1 compared to healthy individuals. Dysregulation of the PD-1 signaling pathway contributes significantly to the inflammation perpetuated in these patients.6,11,12 Similarly in UC, approximately 40% of the infiltrating T cells in the lamina propria in the colon express PD-1 with a 2-fold increase of T cells expressing PD-1 in the blood compared to healthy individuals.13 The immune system of individuals with either of these diseases looks distinctly different than the regulated homeostatic state of a healthy individual.
Targeting the PD-1 with an agonist antibody represents a promising approach to regulate hyperactivated T cells expressing PD-1 in a disease. Preclinical data also suggest they may be more effective in preventing the progression of RA.12 Further understanding of the biological underpinnings of disease pathology and the impact of checkpoint agonism may help to identify indications or patient populations most likely to have deeper or more durable responses to checkpoint agonism, potentially significantly improving patient outcomes.
3. Focus On The Importance of A Cross-Disciplinary Development Approach
Consider a cross-disciplinary development approach that includes antibody optimization, clinical development implications, commercial unmet need, and ultimately, the impact on overall portfolio or corporate strategy.
On the optimization side, aim to engineer targeted antibodies with defined antigen-binding specificities and the right binding affinities required to mediate therapeutic effect. Carefully select optimized antibody candidates that meet numerous design criteria, including long-term stability,14 high solubility,15 low viscosity, appropriate expression level,16 and ability to be formulated at high enough concentrations for convenient patient administration.
These considerations are further informed by a robust understanding of disease biology and future standards of care to inform indication selection and optimal clinical trial design. Commercial considerations, such as unmet need and competition, along with overall impact on long-term corporate strategy, also play key roles in the overall development strategy.
Potential Game-Changing Approach That Targets Multiple Distinct Immune Mechanisms
Targeting co-inhibitory pathways to engage the natural endogenous immune regulatory mechanisms have the potential to not only restore immune balance but also modify a wide range of autoimmune and inflammatory diseases across numerous therapeutic areas.
Currently, there’s a considerable amount of research and development underway to better understand checkpoint agonists and their therapeutic potential. By leveraging the latest immunological and clinical research, drug developers aim to optimize inhibition of inflammatory signaling and modify or prevent disease progression.
As we continue to evolve our understanding of how to regulate our body’s immune system, immune cell modulators may prove to be a key to restoring homeostasis as a treatment for serious, debilitating autoimmune and inflammatory diseases.
References
- Zhang Y, Zheng J. Functions of Immune Checkpoint Molecules Beyond Immune Evasion. Adv Exp Med Biol. 2020;1248:201-226. doi:10.1007/978-981-15-3266-5_9
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887-3895. doi:10.1002/j.1460-2075.1992.tb05481.x
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi:10.1126/science.aar4060
- Paluch C, Santos AM, Anzilotti C, Cornall RJ, Davis SJ. Immune Checkpoints as Therapeutic Targets in Autoimmunity. Front Immunol. 2018;9:2306. Published 2018 Oct 8. doi:10.3389/fimmu.2018.02306
- Philips EA, Liu J, Kvalvaag A, et al. Transmembrane domain-driven PD-1 dimers mediate T cell inhibition. Sci Immunol. 2024;9(93):eade6256. doi:10.1126/sciimmunol.ade6256
- Tuttle J, Drescher E, Simón-Campos JA, et al. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis. N Engl J Med. 2023;388(20):1853-1862. doi:10.1056/NEJMoa2209856
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813-824. doi:10.1093/intimm/dxm057
- Parmley S, Szlyk B, Frank RT, et al. Optimizing PD-1 agonist signaling with membrane proximal binding of rosnilimab, a clinical stage PD-1 agonist IgG1 antibody. Poster presented at: European Crohn’s and Colitis Organisation (ECCO) Annual Meeting; February 21-24, 2024; Stockholm, Sweden. Abstract available at: https://doi.org/10.1093/ecco-jcc/jjad212.0296
- Suzuki K, Tajima M, Tokumaru Y, et al. Anti-PD-1 antibodies recognizing the membrane-proximal region are PD-1 agonists that can down-regulate inflammatory diseases. Sci Immunol. 2023;8(79):eadd4947. doi:10.1126/sciimmunol.add4947
- Rosnilimab HV Phase 1 data. Data on file with AnaptysBio, Inc.
- Canavan M, Floudas A, Veale DJ, Fearon U. The PD-1:PD-L1 axis in Inflammatory Arthritis. BMC Rheumatol. 2021;5(1):1. Published 2021 Jan 11. doi:10.1186/s41927-020-00171-2
- Guo Y, Walsh AM, Canavan M, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One. 2018;13(2):e0192704. Published 2018 Feb 28. doi:10.1371/journal.pone.0192704
- Roosenboom B, Horjus Talabur Horje CS, Smids C, et al. Distribution of mucosal PD-1 expressing T cells in patients with colitis of different etiologies. Scand J Gastroenterol. 2021;56(6):671-679. doi:10.1080/00365521.2021.1906316
- Kuzman D, Bunc M, Ravnik M, Reiter F, Žagar L, Bončina M. Long-term stability predictions of therapeutic monoclonal antibodies in solution using Arrhenius-based kinetics. Sci Rep. 2021;11(1):20534. Published 2021 Oct 15. doi:10.1038/s41598-021-99875-9
- Zarzar J, Khan T, Bhagawati M, Weiche B, Sydow-Andersen J, Alavattam S. High concentration formulation developability approaches and considerations. MAbs. 2023;15(1):2211185. doi:10.1080/19420862.2023.2211185
- Mathias S, Wippermann A, Raab N, et al. Unraveling what makes a monoclonal antibody difficult-to-express: From intracellular accumulation to incomplete folding and degradation via ERAD. Biotechnol Bioeng. 2020;117(1):5-16. doi:10.1002/bit.27196
About The Author:
 Martin Dahl, Ph.D., is senior vice president, research, at AnaptysBio, Inc., a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics. He has more than 25 years of experience in drug discovery and research. Prior to Anaptys, Dahl was global head, immunology network at Takeda Pharmaceuticals, where he led immunology programs applicable to gastro-intestinal diseases, immuno-oncology, neuroinflammation, autoimmunity, and respiratory illnesses. He earned his Bachelor of Science degree at The University of California, Irvine and his Ph.D. at The University of Tokyo, where he studied under one of the foremost experts in cytokine biology, Ken-Ichi Arai. Dahl completed his postdoctoral training at Stanford University in the Department of Pediatrics, where he studied the roles of pulmonary dendritic cells and T cells in respiratory viral infections and how they contribute to chronic airway diseases such as asthma.
Martin Dahl, Ph.D., is senior vice president, research, at AnaptysBio, Inc., a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics. He has more than 25 years of experience in drug discovery and research. Prior to Anaptys, Dahl was global head, immunology network at Takeda Pharmaceuticals, where he led immunology programs applicable to gastro-intestinal diseases, immuno-oncology, neuroinflammation, autoimmunity, and respiratory illnesses. He earned his Bachelor of Science degree at The University of California, Irvine and his Ph.D. at The University of Tokyo, where he studied under one of the foremost experts in cytokine biology, Ken-Ichi Arai. Dahl completed his postdoctoral training at Stanford University in the Department of Pediatrics, where he studied the roles of pulmonary dendritic cells and T cells in respiratory viral infections and how they contribute to chronic airway diseases such as asthma.
