QuanLynx Software Advances ADME/PK Screening

Very early pharmacokinetic and ADME (absorption, distribution, metabolism, and excretion) studies have taken on a "combinatorial" character of their own: In vitro methods are replacing animals; even when animals are used, preliminary studies use "cassettes," or panels of drugs administered simultaneously. Managing information from such studies requires software that reflects this complexity while accommodating high instrument sensitivity.
QuanLynx, a module now offered with Micromass's MassLynx software family, automates unattended multiple reaction monitoring (MRM) and selected ion monitoring (SRM), two screening methods enabled by modern mass spectrometry. MRM is typically carried out on complex tissue samples where animals have been subjected to a cassette, or panel, of discovery-stage compounds. Since such samples are complex and contain very low levels of drugs and metabolites, MRM is limited to triple quarupole or MS/MS instruments. SIR, sometimes referred to as the "poor cousin" of MRM, is adequate for some single-drug experiments as well as in vitro work. SIR may be carried on a single quad MS. About 70% of preliminary drug screening requires triple quad instruments and thus uses the MRM protocol.
A view from the QuanLynx application wizard.
QuanLynx integrates four distinct processes:
- LI>MS-MS optimization
- Construction of acquisition method and data acquisition
- Construction of quantification method and quantification
- Data reporting
Micromass believes these capabilities will ultimately expedite high throughput ADME/PK screening assays of diverse discovery compounds. "Researchers at pharmaceutical companies have written their own macros that achieve similar automated methods development," McDowall stated, "but QuanLynx is the first professionally coded shrink-wrapped product to be introduced to the market for this explosively growing application."
QuanLynx allows analysts to define an experimental process by selecting options from a logical sequence of dialogue boxes presented in a familiar Microsoft Windows NT environment. The software then automatically determines the optimum MS parameters for each compound before quantitation. QuanLynx can be implemented on Micromass's Quattro II, Quattro LC and the new Quattro Ultima tandem quadrupole instruments and on the Waters ZMD single quadrupole running under MassLynx 3.3 (or higher).
High-Throughput: The Name of the Game
Because of a greater focus on the survival of drug candidates up to and beyond first testing in humans, stability, toxicity, adsorption, physiochemical properties, and other ADME/PK assays are being adapted to the high-throughput discovery environment. This would typically involve swiftly assaying hundreds or thousands of potential candidates against each other to optimize efficacy over a series. Generally, absolute measurements are less important than relative ones, high throughput being the ultimate goal. "Very early screens involve testing multiple compounds from a combinatorial library, simultaneously, in the same animal," says Micromass Marketing Director Mark A. McDowall. "Since multiple drugs are being tested together, you need a means of finding interactions. What scientists are really looking for here are relative activities, rather than one big hit. Classic ADME/PK experiments can then be done later on. Like combichem, it's a game of big numbers."

Waters' ZMD 2000 single quadrupole MS.
Those "big numbers" are reduced somewhat by reducing the number of samples tested, an approach known as "n-in-one" dosing, or by single compound dosing with subsequent sample pooling. By either method, the analytical bottleneck is the same—high-throughput quantitation of multiple compounds per sample at reduced concentration levels. One way to attain maximum throughput in this environment is to use efficient, generic HPLC methods. In high performance LC-MS/MS, however, it is not possible to operate with generic MS/MS parameters. QuanLynx eliminates what were traditionally the rate-limiting steps in developing optimized MS parameters for each compound of interest and setting up quantification methods.
Today's ADMD/PK screening involves high throughput quantitative analysis of large numbers of discovery compounds, usually over a time course. Traditional approaches use HPLC-UV or radiolabeling studies. LC-MS is much more selective since it uses a mass signature to identify compounds for identification and subsequent unambiguous quantitation. Mixtures of compounds can therefore be studied in parallel provided that each compound in the cassette has a unique mass signature.
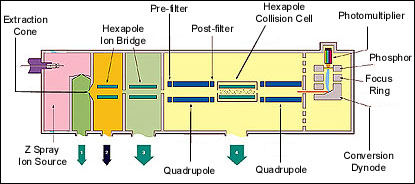
Inside the Quattro Ultima, Micromass's top-of-the-line triple quad instrument.
"We expect QuanLynx tool will allow our customers to make substantial gains in throughput," McDowall said. "QuanLynx leverages the competitive position of our single quadrupole [Waters ZMD 2000], LC-TOF [Micromass LCT] and triple quadrupole [Micromass Quattro Ultima] mass spectrometers with respect to those from our major competitor [Perkin-Elmer] in this application.
QuanLynx is bundled at no additional cost with MassLynx 3.4 (available since August, 1999), MicroMass's established 32 bit Windows NT data handling system for LC-MS and LC-MS-MS.
For more information: Mark A. McDowall, International Marketing Manager, Micromass UK Ltd., Floats Road, Manchester M23 9LZ, UK. Tel: +44 (0) 161 945 4170. Fax: +44 (0) 161 998 8915.
By Angelo DePalma
