PPD Discovery Launches Internet-Based Software for Preclinical Drug Development
First Pass provides integrated technical information needed by those who design and manage development plans. Simultaneously, the program offers real-time clinical, regulatory, and experimental design information. Throughout the design of the preclinical plan, First Pass provides updates on everything going on, for example, how much of a drug candidate is required to complete a particular study. Keeping tabs on materials information prevents studies from being held up while chemists are making more compound.
"First Pass provides parameters and costs along a timeline for how to develop a preclinical plan," said Nancy Zeleniak of PPD Discovery. "The program provides all the technical information required for preclinical research, for example on assays, but the program is also a teaching tool. As you go along it queries and informs you. As you move through it, First Pass continual updates you on the costs. It even alerts you to conflicts when one piece of data doesn't fit with another. First Pass works with you."
Making First Pass available on the Internet allows PPD Discovery to provide updates more easily, Zeleniak said. "Users get access by going to our website from anywhere in their organization. Having their information on one secure location allows easier sharing of data among members of the preclinical team. Let's say a tox study has to be re-run. All a user has to do is put in the new information and First Pass will indicate how much more it will cost and how it will impact your timeline."
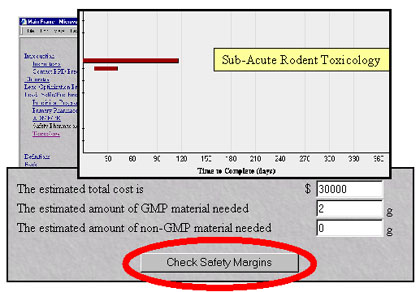
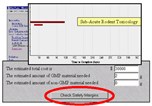
A First Pass preclinical plan elucidates study time, amount of compound required for the plan, and the estimated cost. Users can also obtain an estimate of the safety built in to preclinical programs.
"Traditional project management tools fail to provide detail for understanding technical, design, and information requirements that make preclinical projects successful," said Brad Brown, vice president of research at PPD. "First Pass helps a discovery team understand what steps are on the critical decision path to nominating a drug candidate, and what technologies may be ancillary to drug candidate selection."
First Pass program can be used to identify preclinical studies necessary for any phase of a clinical program, from pre-Investigational New Drug (IND) through New Drug Application (NDA) submissions. The program provides an estimated cost for a proposed preclinical plan, including the amount of Good Manufacturing Practice (GMP) and non-GMP material required for selected studies. First Pass exposes potential conflicts from task interactions or between the preclinical and proposed clinical development plans. In addition, the program delivers a Gantt chart that displays the necessary parameters in a preclinical program, including chemistry, primary pharmacology, safety pharmacology, absorption, distribution, metabolism, excretion, and toxicology. Gantt charts provide a visual definition of the rate limiting steps in the early development stages while illuminating the end points that facilitate project flow.
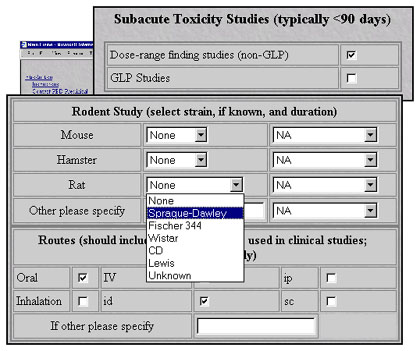
After reviewing informational text from popup windows, users proceed to forms that capture experimental details.
PPD Discovery is the drug discovery arm of of PPD Inc., which is composed of three companies. After preclinical activities PPD Development takes over, taking a project from Phase I to Phase IV (consumer programs). PPD Virtual offers consulting services for virtual drug development. PPD, Inc. employs more than 3,500 professionals in 47 offices in 19 countries around the world.
For more information: Nancy Zeleniak, PPD Inc., 3900 Paramount Parkway, Morrisville, NC 27560. Tel: 919-462-4088. Fax: 919-462-4510.
By Angelo DePalma
