Optimizing Compound and Plate Management
Dave Kniaz, Emax Solution Partners
Executive Summary
The Compound Management Challenge
Bottlenecks and the "Hurry-Up-and-Wait" Syndrome
Compound Management: The Hub of Drug Research
A Typical Screening Procedure
Inventory Management Software: The Missing Link
OPTIMA Substance Manager: The Compound Management Solution
A Driver for Process Improvement
Open and Integrated Operations
Conclusions
Executive Summary (Back to Top)
This article explains how substance and plate management software addresses key bottlenecks in drug discovery programs generated in great part by high throughput technologies. It demonstrates how the software can be used to reduce cycle time, manage a growing compound inventory, and safeguard promising drug leads.
In drug research, the more targets you develop, and the more compounds you create and screen against those targets, the better your chance is of finding the next blockbuster product. Toward this end, pharmaceutical companies have invested in advanced technologies to increase production and screening volumes.
But with this increase comes the challenge of managing millions of compounds, assays and associated data. Bottlenecks in production are causing severe delays between researcher screening requests and compound fulfillment. Cycle times are too long.
Additionally, the increase in inventory increases the chances that a potential blockbuster lead may get lost in the shuffle. Losing a screened substance equates to losing a minimum ascribed value of $10,000, a figure that does not include the millions of dollars in lost sales of a would-be blockbuster drug.
How can drug research companies reduce cycle time, gain a better handle on their growing and dynamic inventories, and free bottlenecks? They can do so by addressing critical bottlenecks in drug discovery pro-grams using plate and substance management software. It is the necessary component that will link end users into the high throughput discovery process and support the just-in-time supply, management and tracking of substances.
This paper presents many of the challenges faced by drug research companies and explains how substance management software can be used to optimize compound and plate management processes, reduce cycle times, and ensure that promising candidates make it through the discovery maze, ultimately supporting a tenfold increase in throughput.
The Compound Management Challenge (Back to Top)
Over the past several years, the nation's top pharmaceutical companies have been modifying their drug discovery programs to take advantage of new technologies that make it possible to select targets at a molecular and genetic level, develop new compounds at a faster rate, and increase the number of targets and compounds that can be screened at a given time. They include:
- Genomics. The genomics and biotechnology era has exponentially increased the number of possible targets for drug discovery. Through genomics, drug companies can discover and validate new disease targets that can be used in screening programs for lead identification. New technologies are expected to expand the number of targets from 500 to 10,000 per year.
- Combinatorial Chemistry. The more compounds a company has, the better the chances are that one will hit the bull's-eye and become a new drug. Combinatorial chemistry allows new organic molecules to be turned out by the yard, dramatically increasing the number of chemical compound combinations used to screen against diseases, from tens per week to tens of thousands per week.
Robotics and High Throughput Screening. Major advances in high throughput screening technology and storage and retrieval robotic systems have increased screening throughput levels from 10,000 compounds per year to levels approaching 100,000 compounds per day. Research centers have combined these new point solutions with aggressive strategies for acquiring new substance entities from discovery collaboration partners, resulting in an exponential increase in the number of discrete substances that must be managed.
Bottlenecks and the "Hurry-Up-and-Wait" Syndrome (Back to Top)
High throughput drug discovery is much like a just-in-time manufacturing process. Companies are finding that major bottlenecks occur not in the machinery workload capability, but rather in logistics management—finding, preparing, and staging the compounds to put into assays, and then analyzing the data. They face the "hurry-up-and-wait" syndrome: huge productivity capabilities in discrete sections of the overall discovery process are followed immediately before or after by an extremely low-productivity task (see Figure 1).
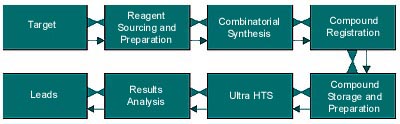
Figure 1
Compound Management: The Hub of Drug Research (Back to Top)
In drug research, the compound management operation is the "hub" of activity and the "bank" for one of the company's most valued assets: new chemical entities (see Figure 2). The compound management function services the research scientists throughout the discovery process by managing inventory and fulfilling researcher requests. Its ability to deliver predictive, easy-to-access, cost-effective, just-in-time service to the research staff is an operational necessity.
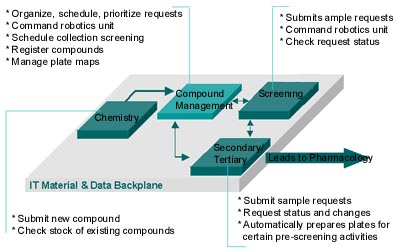
Figure 2
Providing just-in-time fulfillment of researcher requests for substances while tracking and maintaining the rapidly growing inventory of millions of compounds is a complex task. Substances are prepared and stored in plates: specialized storage and transport containers that house 96, 384, or 1536 discrete cells of unique compounds.
Every cell may contain the same substance or one plate may represent a collection of the same substance that will undergo a variety of operations. Regardless, each substance must be managed and tracked throughout its life cycle and its associated information shared among scientists and research workers. Every plate, the materials in each plate well, the parent-child relationships, and the critical information that goes along with each plate must be maintained and tracked with a high degree of accuracy and without creating bottlenecks in the discovery workflow.
Equally important are the logistics management and the referential link between compound and plate/well position information. In most discovery research operations today, this referential link is made by comparing the original plate map (created for the parent plate) to the template used for the test plates, following through on associating the creation of replicates, or multiple doses. In many cases, this involves transferring information between multiple work groups and systems. Even a slight error can result in the incorrect assignment of compounds to well positions. Since 99% of the data is negative, such an error could inadvertently eliminate large blocks of compounds from consideration with little probability that the error would be detected. More importantly, the errors could result in a failure to confirm a potentially viable drug candidate.
A Typical Screening Procedure (Back to Top)
The nature of this logistics management challenge can be more easily appreciated by reviewing one relatively conservative strategy for ultra-high throughput screening (UHTS) of five hundred 96-well plates per day in 10 screening assays:
A research compound dispensary must first select from inventory (or retrieve from pre-prepared sets of combinatorial chemical synthesis plates) 48,000 substances.
These substances are used to prepare plates and plate maps, which identify the position of each substance on each plate.
Typically, a parent plate is produced from which multiple child plates are produced as identical, diluted copies that are used as sources for testing. At least 10 sets of child plates are produced from 10 assays. Sufficient extra copies are produced to accommodate confirmation assays, as well as extras for distribution to other groups within the research program. If 20 child plates are produced from each parent, the result is nearly 1 million discrete test samples that must be tracked.
The plates assigned to each assay must then be broken down into test plates to add controls and blanks. These are processed through the assays, which typically include the addition of reagents, inhibitors, etc., often handled by automated UHTS robots. Plates are read by a variety of methods producing blocks of raw counts or optical density data. The data is "crunched" down to percent inhibition or percent control results, and possibly further processed to produce dose response curves.
Those compounds found to be active (generally around 1%) are usually confirmed by re-testing. This is done through a complex process of cherry picking the active compounds from each assay and creating new plates to be run through the HTS/UHTS process again.
The end result is that more than a half million discrete samples are processed and yield results data each day. Those results must be linked back to the original compound data twice, first to identify active compounds in the first pass, then after cherry picking and processing confirmation data.
Inventory Management Software: The Missing Link (Back to Top)
The number of compounds produced and registered within drug research and the assays required of those compounds exceed the ability of most management systems and compound distribution processes. Compound overload is requiring research executives to establish new business imperatives for their discovery operations, such as efficient supply chain and inventory management processes that serve the drug discovery enterprise throughout each substance's life cycle. As their counterparts in the manufacturing industry have learned, pharmaceutical companies will not realize the true potential of automated operations until they integrate equally sophisticated supply inventory and logistics management with the machinery.
Compound and plate management software is needed as a central system to link end users into a high throughput discovery process and support the just-in-time supply, management and tracking of substances. It is the missing component that will streamline and automate substance registration, requesting, inventory management, and tracking processes.
OPTIMA Substance Manager: The Compound Management Solution (Back to Top)
OPTIMA Substance Manager is an integrated system that provides the researcher with tools and information to manage the entire life cycle of proprietary new chemical entities (NCEs) and libraries. It streamlines the compound preparation and inventory management information at the scientist's desktop. Through electronic inventory management, including bar coding, and integration with robotics, OPTIMA facilitates plate preparation and tracks plates and plate information throughout their life cycle, eliminating information and material bottlenecks (see Figure 3).
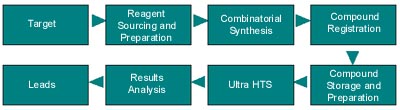
Figure 3
From the arrival and registration of the chemical entity at the dispensary, through the creation, storage, and dispensation of microtube trays, deep-well parent plates, child plates and test plates, OPTIMA eliminates the chance for errors when associating plate/well position information with a compound entity. It helps ensure that compounds are never lost, even when researchers place multiple compounds in a single well.
Using OPTIMA, research operations can achieve a quantum leap in the number of compounds that can be synthesized and analyzed by each researcher by a factor of more than 100 and can eliminate the unnecessary processes that take away from researcher innovation and productivity. Researchers can focus 35–50% more of their work time on high-value scientific activities instead of managing materials logistics.
A Driver for Process Improvement (Back to Top)
Embedded with industry best practices, OPTIMA Substance Manager helps pharmaceutical companies drive business process improvement by simplifying substance registration, tracking, fulfillment and inventory management. OPTIMA supports the entire substance management business flow, including the following business processes:
- Compound and Plate Tracking. Whether plates are used for HTS/UHTS, secondary screening or for shipment to external laboratories for testing, OPTIMA keeps a comprehensive record of the location of each plate (or any type of container) throughout a substance's life cycle. OPTIMA simplifies the searching process by enabling scientists to search for substances by availability, location, container type, parent-child relationships, and freeze/thaw cycles.
- Compound Receiving and Storing. With OPTIMA, you can receive new vials or plates containing samples and assign them to storage locations, integrate with lab balance equipment to obtain tare and gross weight information, and prepare new samples for retrieval from automated or manual storage and retrieval systems.
- Plate Preparation. OPTIMA automates the plate preparation process, enabling you to prepare plates from vials, solubilize master plates and prepare replicate plates via robotics integration, and combine 96-well plates into 384- or 1536-well plates. You can also automate data file transfers to robotic equipment such as Hamilton, Tecan, and Zymark in configurable file formats, to support plate preparation functions. OPTIMA supports user configurable plate formats including validation of plate position information within 96-, 384-, or 1536-well plates, and tracks freeze/thaw cycles for plates and plate parent-child relationships. Without such a system, researchers typically prepare plates manually and enter information into a local database, spreadsheet, or standalone system. With OPTIMA, scientists can check the status of their requests real-time. They can look at the item level and see whether a particular item is available for distribution or in sufficient quantity.
- Compound Request Submission and Fulfillment. With OPTIMA, plate requests can be submitted for high throughput screening and individual sample requests can be submitted for secondary and tertiary screening. When it's time to fulfill a request, compound management personnel can fulfill plate requests from individual powder/solution sample requests or plate replication and dilution functions via robotic automation, fulfill custom plate requests through cherry picking functions, or single sample requests via laboratory balance integration. Plate map data can be submitted to results management systems (e.g., ActivityBase), and picklist reports generated to identify locations of source containers. OPTIMA also provides requester search capabilities, allowing you to search by requester name, request number, request type, recipient, substance identifier, and project protocol (see Figure 4).
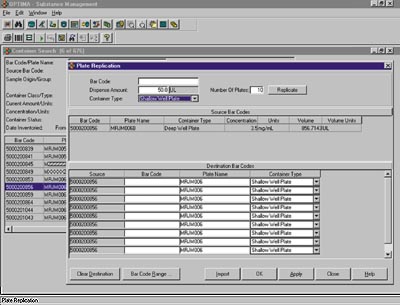
Figure 4
Open and Integrated Operations (Back to Top)
Information technology management will reap business value from OPTIMA's open integration and interoperability. Its component-based system supports both a Windows user interface and a web-enabled, Java-based application approach. Based on a scalable and extensible architecture, the technology supports multi-user, multi-platform, and multi-site configurations and operates with the leading corporate databases.
OPTIMA gateways provide for integration and interoperability with automated container storage systems such as Haystack, Lektriever, or RTS Thurnall's automated stores, as well as with a wide range of robotic and automated synthesis instrumentation. This integration manages the process of maintaining a comprehensive substance collection, in whatever form or format fulfills the constantly changing requirements of the pharmaceutical research effort.
OPTIMA accommodates the needs of a single dispensary or it can function as a truly distributed system, integrating the operations of multiple dispensary groups at as many locations as are required by the needs of a global research organization. This ensures that substances, plates and logistics information are available across an entire multinational organization, helping to optimize the process of new substance discovery.
Conclusions (Back to Top)
Molecular biology, combinatorial chemistry and high throughput screening are dramatically reshaping the drug discovery process. With these advancements comes the challenge of managing millions of discrete compounds.
The ultimate success of these technologies is dependent upon the integration of substance and plate management software into drug discovery programs. OPTIMA links end users into the high throughput discovery process and supports the just-in-time supply, management and tracking of substances. The software optimizes compound and plate management processes by eliminating bottlenecks, reducing cycle times, and increasing throughput at least tenfold, and ensures that promising candidates make it through the discovery maze more reliably and with greater speed.
For more information: Dave Kniaz, VP, Research Informatics, Emax Solution Partners, 18 Campus Blvd., Newtown Square, PA 19073. Tel: 215-325-3700. Fax: 610-325-3782.
