La Jolla Pharmaceutical Advances Stroke Drug Candidate
"Based on positive toxicology results for LJP 1082, we plan to file an IND in the first half of 2001 and will begin clinical trials to assess the safety and efficacy of the candidate," said Steven Engle, chairman and CEO of La Jolla.
Antibody-mediated thrombosis afflicts more than 2 million patients in the U.S. and Europe. Patients have an increased risk of stroke, myocardial infarction, deep-vein thrombosis, recurrent fetal loss, and post-operative complications following cardiovascular surgery. In this autoimmune disorder, antibodies produced by disease-causing B cells target a key blood protein called beta 2 glycoprotein 1, which is involved in the formation of blood clots. When antibodies bind to this protein, inappropriate blood-clot formation is exacerbated. The disease, known as Antiphospholipid Syndrome (APS), is often discovered in younger patients who may lack typical risk factors. Patients usually suffer recurrent, lifelong thrombotic events at more than double the rate of individuals without these antibodies.
New Approach to APS
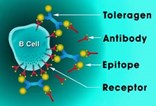
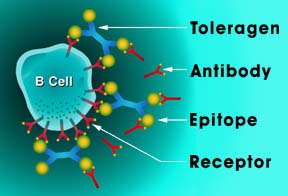
Current therapy relies on anticoagulants, which are difficult to manage, cause serious side effects such as an increased risk of life-threatening bleeding episodes. La Jolla has identified several antibody-mediated thrombosis drug-candidate molecules, called Toleragens, that have been shown to reduce the levels of disease-causing antibodies and their related B cells in a dose-dependent manner in both mouse and rat models.
Toleragens bind to antibody receptors on the cell surface of targeted B cells and send a signal that arrests the production of disease-causing antibodies without suppressing the healthy functions of the immune system. Previously, the La Jolla reported that disease-related antibodies from patients bind to a particular region, called domain-1, on beta 2-glycoprotein 1. These antibodies in APS are believed to increase blood-clot formation by interfering with the natural breakdown of a blood component—Factor Va—that accelerates clotting.
La Jolla Pharmaceuticals' drug discovery effort targets antibodies that cause abnormal clotting.
In La Jolla's animal models of disease, the production of disease-causing antibodies results in a delay in the inactivation of Factor Va, similar to that seen in humans. Using this same Tolerance Technology, the company announced positive clinical trial results earlier this year regarding its lupus Toleragen, LJP 394, which arrests production of antibodies to double-stranded DNA (dsDNA) in lupus patients. Antibodies to dsDNA are believed to be responsible for lupus renal disease, the leading cause of morbidity and mortality in lupus.
In an 18-month Phase II/III clinical trial involving more than 200 patients, drug-treated patients with high-affinity antibodies for LJP 394 experienced one-third as many renal flares and less than one-half as many treatments with high-dose corticosteroids and/or cyclophosphamide as placebo-treated patients.
For more information: Andrew Wiseman, La Jolla Pharmaceutical Co., 6455 Nancy Ridge Dr., San Diego, CA 92121-2249. Tel: 858-452-6600. Fax: 858-623-0649.
