Isis Demonstrates Gene Switch, Stimulating Effects of Chemotherapy
This research represents another first for antisense, which seeks to knock out disease-causing proteins by incapacitating genes that code for them. In this case, Isis showed that the antisense approach could be used to turn a dangerous gene (involved in drug resistance) to a beneficial one.
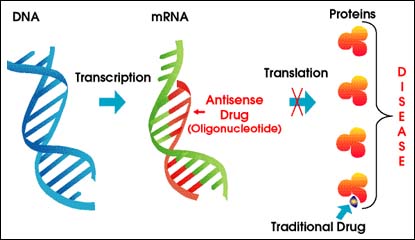
Isis has discovered that antisense can be used to switch dangerous genes into therapeutically useful ones.
The study, "Induction of endogenous Bcl-xS through the control of Bcl-x pre mRNA splicing by antisense nucleotides," was authored by Jennifer Taylor, Qing Qing Zhang, Jacqueline Wyatt, and Nicholas Dean, all of Isis Pharmaceuticals.
Research has shown that alternatively spliced RNA produced from a single gene (Bcl-x gene) produces two proteins (Bcl-xS and Bcl-xL) with opposite functions. Bcl-xS is a promoter of apoptosis, and Bcl-xL is an anti-apoptotic protein. This is an interesting observation in itself, suggesting that the same gene can give rise to proteins with widely different function.
Isis researchers found that by targeting a second generation methoxyethyl (MOE) antisense inhibitor to pre-mRNA from the bcl-x gene, they were able to drive the RNA splicing from one form of Bcl-x to the other. This resulted in a decrease in the Bcl-xL and an increase in Bcl-xS levels in human cancer cell lines. This action, in turn, sensitized cancer cells to apoptotic stimuli and to the cytotoxic effects of chemotherapeutic drugs, therefore increasing their therapeutic effect.
In an editorial analysis of the study appearing in the same issue of Nature Biotechnology, John Reed, scientific director at the Burnham Institute (La Jolla, CA), wrote: "If this trick can be repeated, these findings suggest that it may be possible to switch the phenotypes of other apoptosis-regulating genes. The first steps now have been taken toward the ultimate goal: developing therapeutic approaches that use antisense mediated mRNA splicing to control the cell's own arsenal of—alternately—killing or defensive weapons."
About Isis
Based in northern San Diego County, Isis focuses on antisense and small-molecule drugs for cancer, cytomegalovirus (CMV) retinitis, and inflammatory diseases. Isis's first product, Vitravene (fomivirsen), for treating CMV-induced retinitis in AIDS patients, is being sold in the United States, Europe, and Brazil. In addition, the company has four compounds in human clinical trials. ISIS 2302, an inhibitor of ICAM-1, is in a pivotal quality trial for Crohn's disease, Phase II clinical trials for renal transplant rejection. 2302 is also being explored as an enema formulation for ulcerative colitis, a topical administration for psoriasis, and an aerosol administration for asthma. ISIS 3521, ISIS 5132, and ISIS 2503, that are all in Phase II studies as a treatments for cancer. Several additional compounds are in preclinical development.
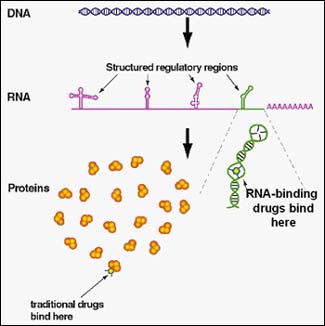
Isis's small molecule development programs also target genes, in this case RNA.
Isis has applied for more than 700 patents, of which 400 have been allowed or granted. In addition, the company has vigorously pursued development partnerships for its own drugs and those of its partners. Isis's key collaborations include:
- Abbott Laboratories: commercial manufacturing and antisense target validation
- AstraZeneca Pharmaceuticals: antisense cancer drug research and development
- Avecia LifeScience Molecules: clinical supply manufacturing
- Boehringer Ingelheim Research: cell adhesion.
- CIBA Vision Worldwide: distribution of Vitravene
- Elan Corp.: development of oral antisense drugs
- Merck & Co.: Hepatitis C.
- Novartis: antisense cancer drugs.
- ProtoGene Laboratories: rapid through-put synthesis of oligonucleotides.
- Rhône-Poulenc Rorer: antisense target validation.
For more information: Jane Green, Senior Director, Investor Relations, Isis Pharmaceuticals, Carlsbad Research Center, 2292 Faraday Ave., Carlsbad, CA 92008. Tel: 760-931-9200.
By Angelo DePalma
