Insights From The Non-Opioid Pain Therapeutics Summit: Human-Centric Translational Models And The Reawakening Of Pain Pipelines

By Ray Dogum, Chief Editor, Drug Discovery Online

Last week, I attended Hanson Wade’s inaugural Non-Opioid Pain Therapeutics Summit in Boston. The event brought together a diverse group of scientists, clinicians, and drug hunters all focused on one thing—developing safe, effective pain medicines without the addictive properties associated with opioids such as oxycodone and fentanyl.
Mapping the Complexity of Pain to Guide Discovery at the Molecular Level
Pain is something we all experience. The manifestation of pain may be the most complex human condition, with each person experiencing it in a uniquely different way. When you accidentally dislocate a joint, burn your fingertips, or stub your toe—you feel pain.
For many, pain arises from more complex pathologies, such as the relentless nerve pain of diabetic neuropathy, the deep bone aches of metastatic cancer, or the inflammatory flares of rheumatoid arthritis. Additionally, the response to therapies can vary dramatically among different patient populations.
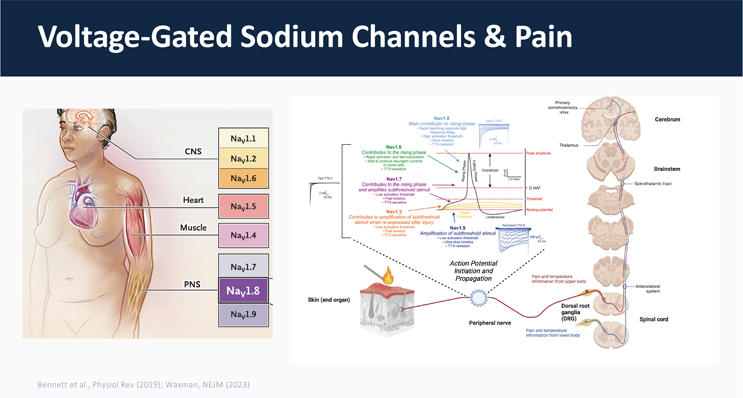
Slide on Voltage-Gated Sodium Channels & Pain, shared by Alexander Chamessian, Assistant Professor, Washinton University in St. Louis. Figures from: Bennett et al., Physiol Rev (2019), Waxman, NEJM (2023).
At the molecular level, pain signaling is shaped by voltage‑gated sodium channels—NaV1.7, NaV1.8, and NaV1.9—which together tune nociceptor excitability. NaV1.7 sets the threshold for action‑potential initiation, NaV1.8 drives propagation and repetitive firing, and NaV1.9 biases neurons toward ongoing activity.
Together, they determine how peripheral pain signals are transmitted to the brain. These NaV subtypes have become high‑value drug targets across the biopharmaceutical industry.
A Landmark Moment: Vertex’s JournaRx (suzetrigine)
Everyone I talked to at the event expressed major gratitude for Vertex’s Journavx (suzetrigine), which was approved by the FDA for treating moderate to severe acute pain. Journavx is notable for being the first and only non-opioid oral highly selective NaV1.8 pain signal inhibitor and the first new class of pain medicine in over 20 years.
After approval in 2025, Vertex has given other companies the confidence to pursue research in alternative pain medicine targeting NaV1.8 as well as other voltage-gated sodium channel inhibitors, effectively derisking this therapeutic area for other companies.
How Acute Pain Can Become Chronic Pain
Acute pain is a significant medical problem that can severely limit daily function and often arises post-surgery, trauma, or injury. Each year, more than 80 million people in the U.S. receive medications for moderate‑to‑severe acute pain, and roughly 40 million of them are given an opioid.
Nearly 10% of patients who start treatment with an opioid continue using it longer than intended, and about 85,000 individuals develop opioid use disorder annually. When acute pain isn’t effectively managed, it can diminish quality of life, contribute to the transition into chronic pain, and place substantial strain on both the health care system and society.
Why Pain Drug Discovery Remains So Difficult

Dr. Marieke Niester, Research Director at the Center for Human Drug Research (CHDR) presenting at the inaugural 2026 Non-opioid Pain Therapeutics Summit in Boston.
For anyone doing drug discovery in the pain space, you know how difficult it is to develop reliable translational models for pain. It’s one of the most difficult conditions to treat as there aren’t many good objective biomarkers for measuring pain.
In most cases, patients are asked to indicate their pain score on a scale from 1 to 10. But as we know, subjective self-assessments of pain make it difficult to determine if a drug is working or if there is a placebo effect at play.
At the Center for Human Drug Research in The Netherlands, Research Director Marieke Niester and her team use PainCarts to measure pain under electrical stimulation, pneumatic pressure, thermal stimulation, capsaicin models, virtual reality pain experience, and other methods in clinical trials.
Even with these sophisticated clinical tools, determining the pathology of pain in order to specifically target it in patients remains a challenge. As Michele Curatolo, Chief Medical Officer of 4E Therapeutics and Professor of Anesthesiology & Pain Medicine at The University of Washinton reminded the audience, “it’s very well-known that psychosocial stress is a modulator of pain, which further confounds clinical trial data. You can’t just measure something in the blood.”
At the conference, Bryan Moyer, Senior Vice President of Discovery at Latigo Biotherapeutics presented his perspectives on the importance of validating new pain targets focusing on human biology to the stregthen confidence of translational studies. Being able to thoughtfully designed discovery and preclinical studies that have the greatest chance of meaningful human safety and effectiveness is critical to developing a successful pain drug.
Simon Westbrook, who’s been in the pain drug development space for over 30 years and scientific founder of Levicept Ltd., suggested, “Patient selection is the key to developing pain drugs.”
Big Pharma’s Interest in Novel Pain Therapies

Speakers from left to right, Chris Adams, Executive Director of Search & Evaluation, Novartis; Darrell Henze, Executive Director, Merck & Co; Asli Sahin, Director of Search & Evaluation, Abbvie; John Markman, Vice President Global Pain, Eli Lilly & Co; Richard Scranton, Vice President of Clinical Development, Vertex.
In a panel focused on big pharma’s perspective, Asli Sahin, Director of Search & Evaluation at Abbvie, shared the company’s ambitious goal of building out a full pain pipeline in 2026, including assets in migraine pain. Darrell Henze, Executive Director at Merck & Co, expressed the importance of having translational biomarkers.
Chris Adams, Executive Director of Search & Evaluation at Novartis, expressed the company’s interest in jumpstarting their pain pipeline. They’re especially interested in candidates with excellent safety and tolerability profiles, emphasizing the importance of quality PK/PD, and thoroughly understanding the biological mechanism of action.
It’s clear that early-stage pain-focused biopharma companies have a real opportunity to partner or license their assets with these organizations. Although there may be some interest in developing combination drugs, clinicians like the ability to “mix-and-match” pain medicine, as needed. So, there needs to be a really valuable reason to pursue a combination approach.
When the event emcee Richard Scranton, Vice President of Clinical Development at Vertex, asked how they’re feeling about licensing and M&A in 2026, the whole panel agreed that they anticipate “a lot of deals” this year.
Novel Approaches & Human-Centric Models
“Human-derived models are not there yet”, said Hongkang Zhang, Senior Director, Pain Therapeutic Lead at Quiver Bioscience. But they are getting closer, as he explained how their AI-driven platform helped them identify Antisense Oligonucleotides as a novel way to modulate NaV1.7, which can avoid existing issues with small molecule inhibitors.
Another interesting approach presented by Alexander Chamessian, Assistant Professor at Washinton University in St. Louis, is to use targeted protein degradation modalities like PROTACs or molecular glues to eliminate (instead of inhibiting) NaV proteins. His lab is conducting early discovery research trying to determine the feasibility of this modality.
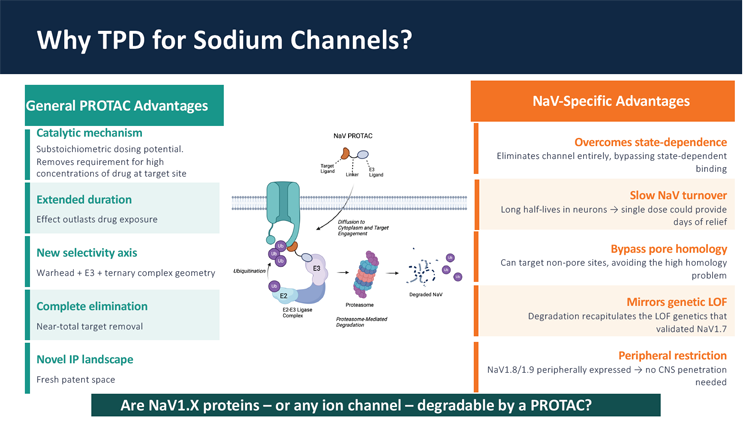
Slide on the advantages of PROTACs targeting NaV channels for pain, shared by Alexander Chamessian, Assistant Professor, Washinton University in St. Louis.

Alexander Chamessian, Assistant Professor, Washinton University in St. Louis presenting at the inaugural 2026 Non-opioid Pain Therapeutics Summit in Boston.
Persistent Challenges & Opportunities Ahead
It’s clear that the opportunities for novel pain treatments continue to reveal themselves, especially for non‑opioids, but the road ahead is far from straightforward. Advances in ion‑channel pharmacology, targeted protein degradation, and human-centric translational models are opening pathways that simply didn’t exist a decade ago.
Together, these challenges and breakthroughs are reshaping what’s possible, signaling a future in which pain therapeutics can be both more precise and less addictive.

Conferences end, but the conversations that matter keep going. Wrapping up the 2026 Non-opioid Pain Therapeutics Summit in Boston, another great event by Hanson Wade. -Ray Dogum, Chief Editor of Drug Discovery Online.
