IBIS Unravels Small Drug-RNA Interactions
The PNAS paper, "Determinants of Aminoglycoside Binding Specificity for rRNA Using Mass Spectrometry," was authored by Richard H. Griffey, Steven Hofstadler, Kristin A. Sannes-Lowery, David J. Ecker, and Stanley T. Crooke of Isis Pharmaceuticals.
Starting with a mixture of aminoglycosides (antibiotics that bind to RNA), investigators developed mass spectrometry (MS) methods that tell which antibiotics bind to suspected RNA drug targets in bacteria. They also measured how tightly and specifically each drug binds while pinpointing the exact binding sites on the RNA targets. Before developing these MS methods, it would have taken years to obtain this level of molecular detail about RNA-binding compounds, but MS allows rapid determination of binding activity, even for multiple targets—a possible boon to high-throughput screening. Ibis expects to be able to screen up to 10,000 compounds per day against multiple RNA targets.
"This valuable advance in drug discovery allows us to sort rapidly through large numbers of RNA-binding molecules to identify the ones that bind to critical regions of our RNA targets," says Ibis Managing Director David J. Ecker. "It has immediate applications for antibacterial and antiviral drug discovery, and could eventually be applied in any therapeutic area."
Although 15 years or so less advanced than antisense technology, Ibis's MS idea complements antisense nicely, at least for RNA binding studies. RNA represents an under-exploited link in the DNA-RNA-protein production mechanism of cells. The earliest drugs interacted primarily with proteins, the end-product of this process; gene studies suggest how drugs might target DNA, the beginning of the chain. As an intermediary in protein synthesis, RNA gives pharmaceutical scientists yet another broad class of targets.
Why RNA?
RNA was thought to be a rather simple molecule, more like DNA than proteins. Recent studies, however, have revealed a surprising intricacy in RNA structure that unlocks new opportunities for the pharmaceutical industry to target RNA with small molecules. Since both proteins and RNA are potential drug binding sites, the number of targets revealed from genome sequencing efforts is effectively doubled when RNA-targeting drugs are considered. Perhaps more importantly, drugs that bind RNA may produce effects that cannot be achieved by binding proteins, and should almost certainly be more specific.
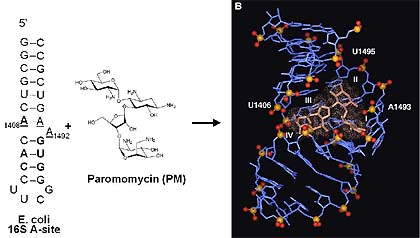
Paromomycin, an antibiotic, binding to the A-site subdomain of 16S ribosomal RNA
In contrast to early-stage drug discovery for traditional protein targets, hitting RNA with small molecules has no major conceptual hurdles to overcome. Proof of principle has already been provided by the success of several classes of drugs obtained from natural sources that have been shown to work by binding to RNA or RNA/protein complexes. These include thiostreptone, the aminoglycoside family and the macrolide antibiotics.
Aminoglycosides are interesting drugs for several reasons, among them their ability to bind sites on RNA that are not prone to mutation. Even after chemical mutagenesis, it is difficult to obtain aminoglycoside-resistant ribosomes. Natural resistance to aminoglycosides is mediated mostly by enzymes that deactivate the compounds, which are transferred among bacterial species via plasmids. Since there are multiple copies of ribosomal RNA genes it is difficult to have resistance mediated by mutations. All this promises effective antibiotics that probably won't become obsolete as organisms adapt.
For more information: David Ecker, Ibis Therapeutics, 2292 Faraday Ave., Carlsbad, CA 92008. Tel: 760-931-9200. Fax: 760-431-2768.
By Angelo DePalma
