FDA grants Fast Track status for Alexion's C5 inhibitor in bypass patients
Enrollment completed in 1,000 patient phase IIB efficacy study
Alexion Pharmaceuticals Inc. (New Haven, CT) announced on Septeber 7 that the U.S. Food and Drug Administration (FDA) has designated Fast Track status for development of its anti-inflammatory complement inhibitor drug candidate 5G1.1-SC for treating patients undergoing cardiopulmonary bypass (CPB) surgery. Alexion has partnered with Procter and Gamble Pharmaceuticals (Cincinnati) on 5G1.1-SC. Fast Track designation provides for expedited review and development.
Alexion has also completed enrollment of about 1,000 patients for a Phase IIb efficacy trial testing 5G1.1-SC in patients undergoing CPB. 5G1.1-SC is a fully humanized single chain monoclonal antibody that blocks inflammation caused by the complement system.
"A significant proportion of patients undergoing cardiopulmonary bypass surgery suffer serious complications, including severe heart and brain damage," commented Christopher Mojcik, senior director of clinical development at Alexion. "Fast Track status should provide us with expedited development and application review for approval of 5G1.1-SC in this patient population. We look forward to working with the FDA and the clinical community to expeditiously bring forward this innovative therapy to the benefit of this large, under-served patient population."
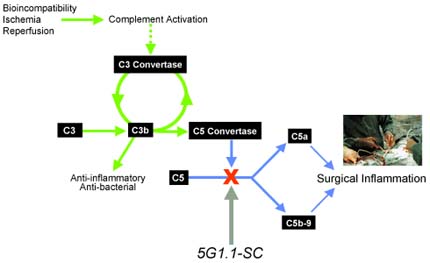
Alexion's 5G1.1-SC complement inhibitor blocks the inflammatory complement cascade responsible for brain and heart injury during surgery.
The Phase IIb multi-center, double-blinded, randomized, placebo-controlled study will examine the safety and clinical efficacy of 5G1.1-SC in approximately 1,000 patients undergoing CBP surgery in nearly 70 centers in the United States. This follows clinical results from Alexion's Phase I/II and Phase IIa CPB studies, published at the end of last year, showing that patients undergoing CPB who received a single dose of 5G1.1-SC showed significant reductions in cardiac damage, new cognitive (brain) deficits, and blood loss relative to placebo-treated patients. These clinical trials are being conducted in collaboration with Procter and Gamble Pharmaceuticals as part of a corporate alliance signed in January 1999.
According to the American Heart Association, in the U.S. in 1996, approximately 500,000 cardiopulmonary bypass procedures were performed with an estimated economic cost of greater than $20 billion. Amongst the more important complications potentially contributing to the costs of bypass surgery are death, heart damage, and stroke.
Alexion's C5 complement inhibitors selectively block the production of inflammation-causing proteins in a process of the human immune system known as the complement cascade.
For more information: Leonard Bell, President and CEO, Alexion Pharmaceuticals Inc., 25 Science Park, New Haven, CT 06511. Tel: 203-776-1790. Fax: 203-772-3655.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online
