Durect Corp. begins construction of commercial manufacturing facility

Located at Durect's headquarters in Cupertino, California
Durect Corp. (Cupertino, CA) has begun construction of a commercial manufacturing facility that is expected to meet Durect's production needs for Phase III clinical trials currently planned for late 2001 and commercial market supply for its lead product, Duros sufentanil. The new facility will comprise approximately 8,000 square feet and will be located at Durect's headquarters in Cupertino, CA. The facility, which includes a state-of-the-art cleanroom and an support for clean assembly and aseptic filling operations, should provide Durect with enough material for getting through trials of its lead product for chronic pain, as well as perhaps providing materials for other pilot/clinical-scale production runs.
Duros sufentanil, for chronic pain, provides an alternative to current therapies and promises improved patient compliance and convenience.
Durect's philosophy is to develop and commercialzie drugs that deliver "the right drug to the right site in the right amount at the right time." Durect's pharmaceutical systems combine technology innovations from the medical device and drug delivery industries with proprietary pharmaceutical and biotechnology drug formulations. These capabilities can enable new drug therapies or optimize existing therapies based on a broad range of compounds, including small molecule pharmaceuticals as well as biotechnology molecules such as proteins, peptides and genes.
Duros technology
Durect's initial portfolio of products combining development-stage drugs with Duros technology, which was licensed for specified uses from Alza Corp. Duros is a miniaturized, implantable, titanium drug delivery pump that can be used for sustained, continuous release.
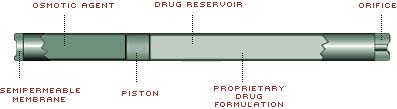
Duros operates like a miniature syringe loaded with a drug inside the drug reservoir. Through osmosis, water from the body is slowly drawn through a semipermeable membrane into the pump by salt residing in the engine compartment. This water fills the pump, which slowly and continuously pushes a piston, dispensing the correct amount of drug out the drug reservoir and into the body. The osmotic engine does not require batteries, switches or other electromechanical parts in order to operate. The amount of drug delivered by the system is regulated by the membrane's control over the amount of water entering the pump and by the concentration of the drug in the drug reservoir.
Duros has several advantages over conventional drug delivery pumps. Duros generates sufficient pressure to deliver highly concentrated and viscous formulations at very high precision (to within 0.001 drop per day). The titanium shell of the Duros system protects the drug formulation from degradation by enzymes and its passage through the body. As a result, the Duros can store and release drugs in the body for up to one year.
Duros can be used for therapies requiring systemic or site-specific administration of a drug. To deliver drugs systemically, the Duros is placed just under the skin, for example in the upper arm, in an outpatient procedure that is completed in just a few minutes using local anesthetic. Removal or replacement of the product is also a simple, quick procedure completed in the doctor's office.
To deliver a drug to a specific site, Durect is developing proprietary miniaturized catheter technology that can be attached to Duros to direct the flow of a drug to the target organ, tissue or synthetic medical structure, such as a graft. Site specific delivery enables a therapeutic concentration of a drug to be administered to the desired target without exposing the entire body to a similar dose. Duros's precision, size, and performance will allow for continuous site-specific delivery to a variety of precise locations within the body.
For more information, contact Schond L. Greenway, director of investor relations at Durect, at 408-777-1417.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online and Pharmaceutical Online
Email: adepalma@vertical.net
