Coley, SmithKline Beecham Ink Infectious Disease Collaboration
Contents
Introduction
Vaccine Enhancement
CpG DNA
Advantages for Immune System Protection/Stimulation
Animal Cancer Models
About Coley Pharmaceutical Group
Introduction (Back to Top)
SmithKline Beecham (Philadelphia) and Coley Pharmaceutical Group (CPG; Wellesley, MA), formerly CpG ImmunoPharmaceuticals, have entered into an exclusive licensing agreement for the development of immunotherapeutic products based on CpG DNA, a broadly enabling technology for immune system stimulation. SmithKline receives a worldwide co-exclusive license to proprietary CpG DNA-based compounds, including CpG 7909, CPG's lead immune stimulant, for use in therapeutic and prophylactic infectious disease vaccines. In exchange, CPG will receive an $8 million up-front payment and possibly another $72 million in license fees, research funding, and milestone payments from SmithKline.
Citing Coley's leading patent position in DNA-based immune stimulation, an emerging field with great potential for new vaccines, Jean Stephenne, president and general manager at SmithKline Beecham Biologicals, stated her belief that CPG's immune stimulants could play "an important role" in her company's current infectious disease programs. For Coley, the SmithKline deal validates its strategy for developing CpG DNA-based products through corporate partnerships.
Over the past nine months Coley has begun three human clinical trials using CpG DNA immune stimulants in vaccines for hepatitis B and influenza, and most recently as an allergy/asthma therapeutic. Additional Phase I/II clinical trials exploring CpG DNA for cancer and allergy/asthma therapy are expected to begin in the coming months. Said Robert Bratzler, Coley's CEO, "By conducting extensive early human testing of our CpG DNA-based compounds, we expect to identify quickly and efficiently the most promising drug candidates for further development across a broad range of therapeutic indications." The clinical trials also give Coley greater leverage in its development deals with larger pharmaceutical partners.
Vaccine Enhancement (Back to Top)
CpG DNA is a broadly enabling technology, encompassing a family of molecules for naturally stimulating and modulating the human immune system. CPG's two lead products, CpG 7909 and CpG 8916, consist of proprietary synthetic DNA sequences that rapidly induce comprehensive, balanced immune responses, providing exceptional therapeutic and prophylactic activity in cancer, infectious diseases, and allergy/asthma models. In preclinical studies, CpG DNA was active when used alone or in combination with disease-associated antigens or antibodies. Subtle variations in CpG DNA sequences can also alter immune responses, providing the potential to create highly specific therapies. These fundamental discoveries in DNA-based immune stimulation are protected by CPG's extensive patent portfolio.
CpG DNA (Back to Top)
CpG DNA, promotes white blood cell proliferation and rapidly activates both cellular and humoral immune responses. CpG DNA was discovered and characterized by Arthur M. Krieg, professor of internal medicine at the University of Iowa and cofounder of Coley Pharmaceuticals. In a 1995 paper in the journal Nature, Krieg showed that specific short stretches of DNA containing cytosine-guanine dinucleotides (CpG) are potent activators of immune cell proliferation and humoral immune responses. Additional research revealed that CpG DNA also stimulates strong cellular immune responses, and that subtle variations in CpG DNA sequences can alter immune responses, providing the potential to create highly specific therapies.
CpG DNA enters a cell and activates genes that effect immune cell proliferation and stimulation. The strong, yet balanced, cellular and humoral immune responses that result reflect the body's own natural defense system against invading pathogens and cancerous cells. These responses have important therapeutic and prophylactic medical applications.
Although relatively rare in human DNA, CpG dinucleotides are commonly found in the DNA of infectious organisms such as bacteria. The human immune system has apparently evolved to recognize CpG dinucleotides as an early warning sign of infection, and to initiate an immediate and powerful immune response against invading pathogens without causing adverse reactions frequently seen with other immune stimulatory agents. By relying on this innate immune defense mechanism, pharmaceuticals based on CpG DNA can capitalize on a unique and natural pathway for immune therapy.
Advantages for Immune System Protection/Stimulation (Back to Top)
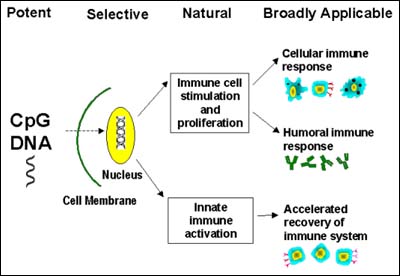
CpG DNA's comprehensive immune activation, and particularly strong cellular immune stimulation, confer exceptional therapeutic and prophylactic responses, potentially providing a new approach to treating cancer and chronic infectious diseases. Certain CpG DNA sequences also selectively redirect inflammatory allergic or asthmatic reactions into harmless immune responses, providing new treatments for such conditions as asthma. Other CpG DNA sequences activate innate immunity, and can potentially be used to help protect against opportunistic infections after cancer chemotherapies or other immunosuppressive treatments.
Compared with other immune stimulants, CpG DNA has the following advantages:
- Potent: Stimulates immune cell proliferation and both cellular and humoral immune responses, providing increased potency and enhanced therapeutic and prophylactic effects.
- Selective: Promotes antigen-specific immune responses, providing specifically targeted immune responses that do not harm normal tissues.
- Natural: Boosts the body's natural immune responses in a balanced fashion, potentially offering safer immune stimulation with fewer side effects.
- Broadly Applicable: Enables development of multiple products for a wide variety of diseases, including cancer, infectious disease, allergy and asthma.
Animal Cancer Models (Back to Top)
Animal studies indicate that CpG DNA, when used with monoclonal antibody or vaccine therapy, can dramatically improve survival in both lymphoma and melanoma models.
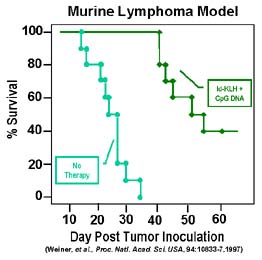
In a mouse lymphoma model, all control mice died by day 32. C3H mice were immunized subcutaneously with a single dose of Id (B cell lymphoma tumor-associated antigen)-KLH plus CpG DNA or were unimmunized. Two weeks later they were challenged with 1,000 38C13 (lymphoma) cells. Survival was followed for 100 days. All mice alive after 55 days remained tumor-free for the entire observation period.
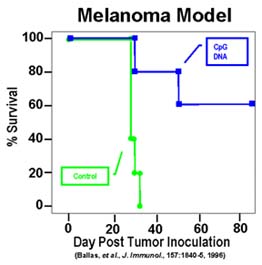
Similarly, CpG DNA injected into mouse melanoma lesions increases protection against tumor growth from 0% to 40%. Mice were injected with 500,000 B16 melanoma cells intraperitoneally on Day 0. Concommitantly, CpG DNA or non-CpG ODN was injected IP on days 0, 3, 7 and weekly thereafter. Survival was followed for 150 days.
About Coley Pharmaceutical Group (Back to Top)
Privately held Coley Pharmaceutical Group develops therapeutic and prophylactic products that harness the immune system to treat cancer, infectious diseases, allergy and asthma. The company believes its products also show promise in accelerating immune system recovery after cancer chemotherapy or other immunosuppressive treatments. Coley was founded in 1997 and is based in Wellesley, MA, USA, with European operations based in Hilden, Germany and Canadian operations in Ottawa. Current investors include TechnoVenture Management, Alafi Capital, and Qiagen N.V.
For more information: Robert Bratzler, CEO, Coley Pharmaceutical Group, 55 William St., Suite 120, Wellesley, MA 02481. Tel: 781-431-6400. Fax: 781-431-6403.
Angelo DePalma
