Big Pharma Licensing Activity Up 62%; Biotech Opportunities Up 102%

The area that has shown the highest growth rate is biotechnology. Since 1992 the top 30 pharmaceutical companies have doubled the proportion of their licensing opportunities that are biotech products. This rapid increase is mirrored, although to a lesser extent, by the rest of the industry.
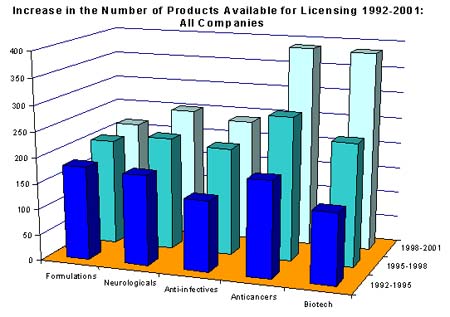
Taking biotech as an example, the graph shows how, between 1992 and 2001, the number of biotechnology licensing opportunities throughout the industry increased from 138 to 386. As a proportion of total licensing opportunities, this represents an increase of 90%. (Copyright 2001 PJB Publications Ltd)
In addition to biotechnology, other areas showing large increases in licensing activity include anticancers, anti-infectives, neurologicals and formulations. As illustrated by the graph above, activity has risen in each of these therapy areas with biotech and anticancer drugs showing the most dramatic increases.
During the period 1992-1995, the top 30 companies sought licensees for nine out of every 100 pipeline drugs. As shown in the diagram below, this figure rose to 14 during 1998-2001. Comparing these figures to the industry as a whole it is clear that industry licenses out significantly more products (22% during 1998-2001) but that this proportion is increasing at a much slower rate compared to the large companies.
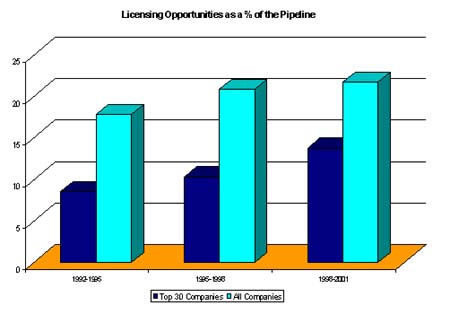
(Copyright 2001 PJB Publications Ltd)
This growth confirms the predictions made by David Scott in Scrip's Practical Guide to Pharmaceutical Licensing, 'Despite the continuing consolidation of the multinational pharmaceutical industry, the growth of specialist drug delivery companies and biotechnology companies has continued apace and has greatly increased the scope for licensing opportunities.'
‘The products being generated by these new companies, coupled with changes in the competitive, patent and regulatory environments, have also meant that major multinationals now see licensing as an essential part of their development strategy. As a result, the amount of licensing activity is increasing both in terms of the number and types of deals being undertaken.'
‘Today, there are probably over 5,000 biotechnology companies, compared with fewer than 200 multinational pharmaceutical companies and approximately 1,000 national companies, many now arising in the newly emerging Eastern European and Asian markets. This means that the number of opportunities to in- and out-license continues to rise.'
Pharmaprojects identifies, on average, 500 new licensing opportunities each year. Valuable sources of information on these opportunities are the increasing number of bio-partnering conferences that take place each year. Pharmaprojects will be reporting on the 9th Annual BioPartnering Europe meeting which takes place from 14-16 October in the QE II Conference Centre in London, England. For more detailed information, please see www.biopartnering.com.
About Pharmaprojects
Pharmaprojects, the leading database tracking pharmaceutical development from early preclinical study through to launch or discontinuation, has 21 years experience as an information provider to the industry. Pharmaprojects uses a fully-searchable application that allows you to pinpoint the specific information you are looking for whether it be comprehensive drug profiles, a competitor's pipeline or licensing opportunities. Updated monthly, the data is also available weekly via online hosts PJB Publications, Dialog and Datastar, Ovid Technologies and STN International.
Pharmaprojects R&D Time-Lines is the definitive R&D benchmarking resource tool that allows you to measure the probability of a drug's success through each phase of development and estimate how long it will take a drug to reach the market.
The Pharmaprojects team is available for comment or to provide presentations on trends in pharmaceutical R&D; for details, please contact Ian Lloyd, Managing Editor.
Scrip's Practical Guide to Pharmaceutical Licensing, is a step-by-step guide that brings together the most up-to-date information you need to plan a successful licensing strategy to achieve revenue growth and stimulate innovation. For more information on this report please contact the Customer Service helpdesk on Tel: +44 (0) 20 8332 8965/6 or e-mail custserv@repsinfo.demon.co.uk
About PJB Publications
PJB Publications, established in 1976, is an independent publishing company of business-to-business information. Specialist areas of knowledge include pharmaceuticals on which it publishes the twice-weekly newsletter Scrip, medical devices and diagnostics, biotechnology, veterinary, crop protection, agribusiness, clinical research and regulatory affairs.
