Antex Biologics Patents Helicobacter Pylori Vaccine
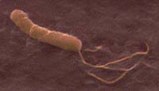
The Centers for Disease Control and Prevention (CDC) estimates that two-thirds of the world's population is infected with H. pylori. Twenty-five million Americans are treated for ulcers each year. H. pylori was discovered in 1982. Science quickly established a correlational, then a causal relationship, between the bacterium and stomach ulcers. Despite statistics showing that between 90% and 95% of ulcers were caused by Helicobacter, for years gastroenterologists resisted (and some still do) the notion that a disorder that used to require life-long palliative treatment could now be cured in a few weeks through antibiotics.
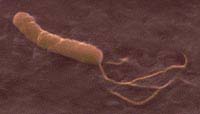
Helicobacter pylori hide in the stomach's mucosa, where they are believed to cause ulcers and possibly stomach cancer.
Ulcers cost an estimated $6 billion annually in medical bills and lost workdays. Currently, no vaccines exist to prevent this disease. Each year one million Americans are hospitalized from complications caused by ulcers, 500,000 adults are treated for peptic ulcer disease, 40,000 patients undergo emergency surgery, and 6,000 die, according to the CDC. Infected people are also six times as likely to develop stomach cancer.
Two forms of cancer are linked to infection with H. pylori, the only bacteria classified by the World Health Organization as a class I carcinogen. The American Cancer Society estimates that more than 25,000 new cases of stomach cancer and over 13,000 deaths per year occur in the United States. How many are directly caused by Helicobacter is anyone's guess.
Although antibiotics are now recognized as a cure for ulcers they do not prevent reinfection with H. pylori, which occurs quite readily among typically asymptomatic family members. A vaccine, administered several times over a period of three or four weeks, might confer immunity for life. Interestingly, this vaccine might be administered to those already infected since it helps the body clear H. pylori as well as preventing infection.
In January 1999, Antex announced completion of its Phase I clinical trial for the Helicobacter vaccine, finding that the orally administered, whole-cell (plus adjuvant) vaccine was well tolerated and produced both systemic and mucosal immunity, especially in the stomach lining. Earlier animal studies showed the vaccine prevented infection in animals and cleared existing infections.
Technology Platform
Antex uses nutriment signal transduction (NST) to develop products for treating a broad spectrum of bacterial infections. NST regulates bacterial growth and/or virulence by reproducing, in the laboratory, critical environmental signals for bacterial growth. In other words, it results in antigenically enhanced "super-bugs" resulting in more potent, clinically relevant inactivated whole cell vaccines. There's another side to this, which should be of great interest to pharmaceutical developers. Some of the newly expressed virulence factors of NST-grown bacteria are metabolic proteins, which are currently being evaluated as novel drug targets for new antibiotics.
Antex now has eight issued or allowed patents for its NST technology, which include claims to its Campylobacter and Shigella vaccines as well as many other enteric bacteria.
Alliances
It's virtually impossible these days for a small company to bring a vaccine to market. Antex has therefore entered alliances with the top three worldwide vaccine developers to extend its reach into major anti-infective vaccine markets.
In May 1996, Antex and SmithKline Beecham formed a joint venture to develop and commercialize human bacterial vaccines for respiratory, gastrointestinal, and sexually transmitted diseases. This alliance could result in research and development and milestone payments to Antex totaling $30 million, plus royalty payments on product sales. SmithKline Beecham will be responsible for the clinical development, manufacturing and worldwide marketing of human bacterial vaccines resulting from the joint venture.
In 1994, Antex licensed Hin47, a subunit vaccine against Haemophilus influenzae (nontypeable), to Pasteur Mérieux Connaught, which is responsible for developing a vaccine product. Antex will receive milestone payments and royalties based on product sales. This vaccine could not be developed too soon: Otitis media, an ear infection caused by Haemophilus, is a leading infectious disease for infants and children and the bane of parents taking their children to pediatricians. Increasingly resistant to antibiotics, there are no vaccines on the market for this infection. A Phase I clinical trial of the vaccine began in February 1997.
In November 1994, Antex signed a Cooperative Research and Development Agreement (CRADA) with the Department of the Navy to clinically test Antex's vaccine to prevent gastroenteritis and diarrhea caused by Campylobacter, a nasty pathogen that causes "traveler's diarrhea" and infects an estimated 400 to 500 million people annually worldwide. Diarrhea is also a major killer of children in developing countries.
The Navy funded two Phase I clinical trials of the vaccine and is conducting a Phase II trial that began in July 1997 at Fort Detrick, MD.
In August 1996, Antex was awarded a Phase II Small Business Innovation Research (SBIR) grant by The National Institutes of Health (NIH) to develop a vaccine for Chlamydia trachomatis, the most prevalent sexually transmitted disease in the United States. Using Antex's ART technology, a novel surface protein has been identified and is in preclinical evaluation as a subunit vaccine.
For more information: Theresa Stevens, VP of Corporate Development, Antex Biologics., 300 Professional Drive, Gaithersburg, MD 20879. Tel.: 301-590-0129. Fax: 301-590-1251.
By Angelo DePalma
