Small Cells, Big Shifts: Recent Advances In Targeting Small Cell Lung Cancer
By Ashton Curtis, Jack Gordon, Thomas Pataillot-Meakin, and Kasia Koczula, Lifescience Dynamics
Small cell lung cancer (SCLC) is a high-grade neuroendocrine carcinoma, biologically and clinically distinct from non-small cell lung cancer (NSCLC) and historically associated with significantly shorter survival. Accounting for ~15% of lung cancers (vs. ~85% for NSCLC), SCLC is typically classified as limited-stage (LS-SCLC) or extensive-stage (ES-SCLC). Unlike NSCLC, therapeutic progress in SCLC has been limited, with meaningful advances emerging only in the past two years. In this article, we discuss these developments and consider the future treatment paradigm of SCLC.
PD-L1 Therapies: The Current Standard Of Care In SCLC
Currently, four PD-L1 regimens are FDA approved across the SCLC continuum (Figure 1). In 1L ES-SCLC, atezolizumab or durvalumab-based regimens are the standard.1,2 However, adding these agents to chemotherapy only improved survival by roughly two months, with the modest survival benefit of aPD-L1 therapies likely reflecting the immunologically “cold” tumor microenvironment (i.e., not likely to trigger an immune response) in SCLC. Notably, recent advances in 1L ES-SCLC remain modest with maintenance intensification of aPD-L1-based therapy, with the addition of lurbinectedin (DNA-alkylating agent) to atezolizumab further extending survival by around three months beyond chemoimmunotherapy. Following its approval in October 2025, this combination is likely to represent the 1L ES-SCLC standard of care in the near term.
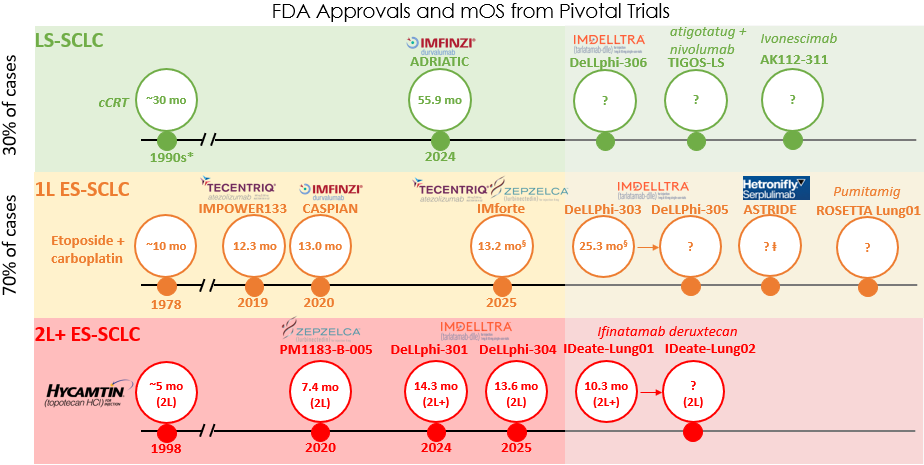
Figure 1. Abbreviations: cCRT (concurrent chemoradiation therapy). Key: *cCRT is not regulated by the FDA. cCRT was introduced in the early 1990s and cemented by the late 1990s–early 2000s. § Overall survival (OS) was measured from point of maintenance treatment (following induction therapy). ⱡ ASTRIDE is an ongoing Phase 3 U.S.-bridging trial to seek FDA-approval. Phase 3 ASTRUM-005 with trials sited in China and the EU yielded a median overall survival (mOS) of 15.4 months. Other notable pipeline assets are ABBV-706 (P3 SEZanne 1L ES-SCLC); ZL-1310 (planned P3 in 1L ES-SCLC); obrixtamig (P1 DAREON-8 1L ES-SCLC); and gocatamig (P1/2 2L+ ES-SCLC).
Moving away from intensification approaches, the identification of selective SCLC biomarkers has enabled new targeted approaches that are showing step-changing efficacy improvements.
Rise Of DLL3 T-Cell Engagers
T cell engagers (TCEs) act as bridges, linking a patient’s T cells to target tumor cells and enabling immune-mediated tumor killing. Tarlatamab, a first-in-class DLL3-targeting TCE, has delivered the first major efficacy milestone in 2L+ ES-SCLC, extending overall survival by about seven months in DeLLphi-301, leading to accelerated U.S. approval in May 2024 (Figure 1).
At ASCO 2025, further encouraging data from the confirmatory DeLLphi-304 trial in 2L ES-SCLC were presented, leading to full FDA approval in November 2025.. However, a class effect of TCEs is cytokine release syndrome, necessitating 48-hour inpatient monitoring, which has posed a headwind to community adoption of tarlatamab; safety data from cohorts with reduced monitoring burden will be critical to facilitate outpatient use.
Tarlatamab may also hold promise in the 1L ES-SCLC setting, as suggested by Phase 1 DeLLphi-303 data presented at WCLC and ESMO 2025. Tarlatamab combined with a PD-L1 inhibitor as 1L maintenance extended overall survival by approximately 12 months versus the current standard. A Phase 3 DeLLphi-305 trial of tarlatamab and durvalumab in 1L maintenance is underway, which may poise tarlatamab for FDA approval in this setting in the near future.
Other DLL3-targeting T cell engagers also in development for SCLC include obrixtamig, gocatamig and alveltamig (biparatopic DLL3 TCE); however, early-stage clinical data show little substantial differentiation from tarlatamab.
Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) selectively bind to proteins on cell surfaces to deliver cytotoxic payloads specifically to tumors, resulting in enhanced targeting over standard chemotherapy. ADCs have redefined the treatment paradigm in other solid tumors such as NSCLC and breast cancer. The most advanced ADC in global clinical development for SCLC is I-DXd (B7-H3 ADC). Data presented at WCLC 2025 from the IDeate-Lung01 trial of I-DXd in 2L+ ES-SCLC demonstrated an encouraging overall survival of 10.3 months (Figure 1), with the ongoing IDeate-Lung02 expected to complete in 2026. Other notable ADCs in development for relapsed ES-SCLC include GSK’227, BNT324, YL201, and ZL-1310.
In terms of 1L ES-SCLC, only two ADCs are under clinical development, I-DXd (IDeate-Lung03) and ABBV-706 (SEZ6 ADC; SEZanne), with no data yet available for ADCs in this line of therapy. However, given that most of these ADCs use a TOPO1i payload, the question arises as to whether ADC re-challenge after 1L ES-SCLC treatment is appropriate.
PD-(L)1xVEGF Bispecific Antibodies
PD-(L)1xVEGF bispecific antibodies have recently gained traction, based on the principle of co-localization of mechanisms of action to increase on-target effects while limiting off-target activity, thereby lowering toxicity and increasing efficacy. BioNTech’s pumitamig (PD-L1xVEGF-A) demonstrated encouraging tumor responses at WCLC 2025 in a Phase 2 trial in 1L ES-SCLC (overall response rate [ORR] 76%; median progression-free survival [mPFS] 6.8 months)3, and interest in this class in SCLC continues to grow with Pfizer in-licensing SSJ-707 and set to initiate a Phase 2/3 trial in 1L ES-SCLC.
The Future Standard Of Care In SCLC
There has been rapid development of new therapeutics in ES-SCLC, led by ADCs and TCEs, with a handful of further FDA approvals expected across settings in the next two years. Though it remains unclear which therapeutic modality will deliver superior overall survival, several companies are now investigating ADC–TCE combinations as the next-gen chemoimmunotherapy with the potential to replace standard etoposide/carboplatin for 1L ES-SCLC. Key trials aiming to validate this approach include Merck’s Phase 1/2 trial of I-DXd and gocatamig and Amgen’s DeLLphi-310 trial of tarlatamab and YL201. We therefore suggest that the future standard in 1L ES-SCLC is likely to move to combination regimens of ADCs and TCEs that have already shown strong single-agent activity in later treatment lines.
References
- https://ascopubs.org/doi/10.1200/JCO.20.01055?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed#sec
- https://www.sciencedirect.com/science/article/pii/S0923753419604497?pes=doc&utm_source=wolterskluwer&getft_integrator=wolterskluwer
- https://news.bms.com/news/corporate-financial/2025/First-Disclosure-of-Global-Interim-Phase-2-Data-for-BioNTech-and-Bristol-Myers-Squibb-PD-L1xVEGF-A-Bispecific-Antibody-Pumitamig-BNT327-BMS986545-in-Patients-with-Extensive-Stage-Small-Cell-Lung-Cancer-Shows-Encouraging-Antitumor-Activity/default.aspx
About The Authors:
 Ashton Curtis, Ph.D., is a business analyst at Lifescience Dynamics with a diverse research background across academic and industry settings. He has extensive experience in conducting CI and market research in oncology, immunology, and rare diseases. Ashton obtained his Ph.D. in structural biology and M.Sci. in pharmacology from University College London.
Ashton Curtis, Ph.D., is a business analyst at Lifescience Dynamics with a diverse research background across academic and industry settings. He has extensive experience in conducting CI and market research in oncology, immunology, and rare diseases. Ashton obtained his Ph.D. in structural biology and M.Sci. in pharmacology from University College London.
 Jack Gordon, D.Phil., is a business analyst at Lifescience Dynamics with experience delivering decision support and strategic insight projects for global pharma clients across solid and hematologic tumor indications, as well as immunology and rare diseases. His research background includes biotech preclinical R&D in areas such as neurodegeneration and expertise in computational biology and single-cell gene expression technologies.
Jack Gordon, D.Phil., is a business analyst at Lifescience Dynamics with experience delivering decision support and strategic insight projects for global pharma clients across solid and hematologic tumor indications, as well as immunology and rare diseases. His research background includes biotech preclinical R&D in areas such as neurodegeneration and expertise in computational biology and single-cell gene expression technologies.
 Thomas Pataillot-Meakin, Ph.D., is a business analyst at Lifescience Dynamics with a doctorate in oncology. He has supported clients over a wide range of oncology, immunology, and early GI therapeutic areas with expertise in competitive intelligence, market research, and market forecasting. His work spans pipeline strategy, conference intelligence, and cross-functional insight generation to inform commercialization and medical strategy.
Thomas Pataillot-Meakin, Ph.D., is a business analyst at Lifescience Dynamics with a doctorate in oncology. He has supported clients over a wide range of oncology, immunology, and early GI therapeutic areas with expertise in competitive intelligence, market research, and market forecasting. His work spans pipeline strategy, conference intelligence, and cross-functional insight generation to inform commercialization and medical strategy.
 Kasia Koczula, Ph.D., is an engagement manager at Lifescience Dynamics, bringing over eight years of consulting experience in the life sciences sector. She holds a Ph.D. in hematological oncology and has worked across a broad range of therapy areas, including oncology, cardiovascular diseases, and rare diseases. Kasia specializes in competitive intelligence, market research, and strategic advisory services, supporting clients with evidence-based insights to drive informed decision-making.
Kasia Koczula, Ph.D., is an engagement manager at Lifescience Dynamics, bringing over eight years of consulting experience in the life sciences sector. She holds a Ph.D. in hematological oncology and has worked across a broad range of therapy areas, including oncology, cardiovascular diseases, and rare diseases. Kasia specializes in competitive intelligence, market research, and strategic advisory services, supporting clients with evidence-based insights to drive informed decision-making.
