Room For Improvement: Reformulating Existing Therapeutics
A conversation with Jed Litwiniuk, MHA, CEO, and Uwe Tigör, MD, CMO, Auxilius Pharma

Heart disease is the leading cause of death in the U.S. Coronary artery disease (CAD) affects roughly one in 20 adults age 20 and older. Its most distinctive symptom is chronic stable angina pectoris (CSAP) — a type of chest pain that is typically fixed to the level of exercise with which it occurs. It is brief in duration, but can worsen over time. This is caused when the heart muscle needs more oxygen but isn’t receiving it — most commonly because of underlying atherosclerotic coronary disease. CSAP usually progresses over time and can lead to heart attacks that cause complete blockage and permanent heart damage. Currently, about 16 million Europeans and about 11 million Americans suffer from chronic angina.
In this Q&A, Life Science Connect’s Morgan Kohler caught up with Jed Litwiniuk, CEO, and Uwe Tigör, CMO, to talk about Auxilius Pharma’s work to improve and reformulate a treatment for CSAP.
What does Auxilius' R&D strategy look like?
Litwiniuk: Our R&D strategy focuses on high unmet-need areas in cardiovascular disease, namely CSAP. We specialize in transforming and enhancing existing medications by optimizing their release profiles to make them more convenient, safe, and effective for patients. Despite major advances in healthcare, many commonly used medications remain poorly formulated, creating opportunities for companies like Auxilius.
In some therapeutic areas, including CSAP, several therapies approved in Europe have never been approved in the United States, limiting American patients’ access to effective treatments with alternative mechanisms of action. Nicorandil, a proven anti-anginal agent, is one such example. For AUX-001, we reformulated the reference drug (immediate-release [IR] nicorandil) — previously dosed two to three times daily — into a once-daily product. Approximately 20 years ago, nicorandil’s Japanese originator, Chugai, planned to introduce the drug in the U.S.; however, following Chugai’s acquisition by Roche, the program was discontinued as Roche exited development of medications in this section of cardiology. As a result, nicorandil has never been approved in any form in the United States.
Does AUX-001 have a unique mechanism of action?
Tigör: It does. Nicorandil is a balanced vasodilator that features a dual mechanism of action: it causes venous and arterial vasodilation and thereby increases blood flow throughout the heart muscle. This reduces what cardiologists call both pre- and afterload on the heart muscle by reducing its oxygen demand.
In a very unique way, nicorandil also conditions the heart muscle to work better within a suboptimal oxygen supply environment, and this “training” (called ischemic pre-conditioning) is thought to be the reason for its long-term cardioprotective effect. This is the very reason why nicorandil remains the only anti-angina medication that reduces the symptoms of CSAP (along with all other anti-angina medications) while also improving the prognosis of the underlying CAD that most CSAP sufferers share as the cause of their angina symptoms.
What did the R&D at Auxilius look like to make AUX-001?
Litwiniuk: AUX-001 was developed and optimized based on regulatory and clinical trial data for IR nicorandil, which has been used in Europe, parts of Asia, Australia, and New Zealand for three decades.
In our preclinical work, we began with in silico modeling to validate the pharmacokinetics and pharmacodynamics (PK/PD) potential and define the optimal release profile for once-daily administration. This was followed by extensive pharmaceutical development to create a new formulation that aligns with this target profile. While this may sound straightforward, it was not. Nicorandil is a chemically unstable and challenging molecule, making it difficult to extend its release profile while maintaining stability. This is why it was originally developed as an immediate-release product, despite the resulting plasma-level fluctuations that are suboptimal for patients. Although details are proprietary, our release-modifying technology enabled us to overcome these challenges.
Prior preclinical work had already validated IR nicorandil, which can be used for any future market authorization filings with the FDA and the EMA. In regulatory terms, the FDA has reviewed and qualified AUX-001 as a 505(b)2 candidate in the U.S. We expect to file the NDA in 2028. In the EU, approval would follow the hybrid pathway, allowing Auxilius to proceed directly from Phase 1 to a single Phase 3 study to demonstrate AUX-001’s efficacy.
Tigör: It helped that IR nicorandil had already demonstrated and validated a dual mechanism of action and preclinical safety as evidenced by its previous approval. For our FDA filing, we will use the data submitted to the EMA during the EU approval of IR nicorandil, plus potentially some additional preclinical studies that were not required at the time of the original submission. In Europe, we will not need to resubmit preclinical data on nicorandil.
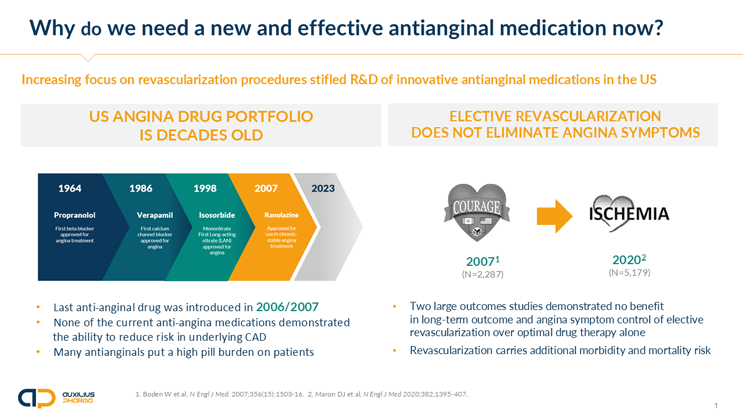
There hasn't been a new CSAP therapy introduced in nearly 20 years. How did Auxilius navigate developing a different therapeutic for CSAP? Why has the creation of a new therapy been lacking all this time?
Tigör: When IR nicorandil was first approved in select countries of the EU and larger Europe between 1992 and 1997 (The Netherlands, U.K., France, Denmark, Portugal, Austria, Switzerland; not in Germany, Italy, or Spain) cardiology was in the midst of changing the treatment of CAD. This new revolutionary approach was invasive catheter-based, intracoronary angiography, and coronary stenting. This technology, along with many fulfilled promises, also carried the hope that chronic drug treatment in CSAP would become a thing of the past and that, with coronary stent-based revascularization within the obstructed coronary artery tree, the symptoms of CAD, namely CSAP, would permanently disappear.
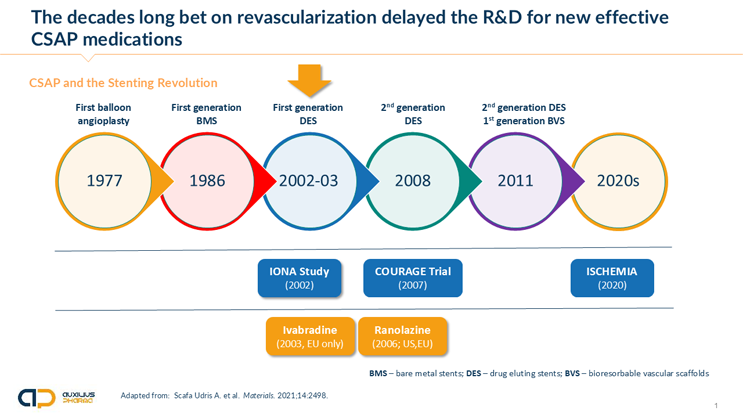
Now, 30 years later, we know that with the onset of CSAP, patients enter a new, much higher risk category. Stenting neither affects the prognosis of these patients nor does it, even after a short improvement, eliminate CSAP symptoms.1,2
The graph below highlights this development and the main clinical trials that were necessary to disentangle cardiology from the — by now — quite entrenched idea that stenting would permanently solve the problem of CSAP.
What makes nicorandil unique among approved anti-angina medications is its ability to not only treat the chronic symptoms of chest pain but also reduce adverse outcomes of the underlying coronary disease, such as cardiovascular events and the frequency of hospital admission and visits to the emergency room — a major cost driver in CSAP. The landmark Impact of Nicorandil in Angina (IONA) Study, which recruited 5,126 people with stable angina, represents one of the largest CSAP studies ever conducted, with approximately 8,500 patient years of data.3
AUX-001 is an optimized once-daily version of IR nicorandil. The original IR formulation must be taken multiple times a day to achieve efficacy. Our Phase 1 data presented at the ESC and AHA last year indicate that this optimization may hold promise for improvements beyond simplifying dosing frequency — namely, in safety and tolerability.
What constraints or roadblocks are there in developing therapeutics for an unmet medical need?
Litwiniuk: Despite pharmaceutical companies providing urgently needed and new therapeutic options, we often see physicians reluctant at first to implement these new treatments when a new medication is introduced. That conservatism is built into the practice of medicine, which relies on clinical experience and the understandable attitude to “do no harm.” Resulting uptake inertia and skepticism are a challenge for any new treatment option. In our case, this relates to a persisting high acceptance for the use of stenting to treat CSAP despite ample evidence to the contrary.
In the EU specifically, European payors often do not appreciate the innovative value of developing new modified-release technologies and view them as marginal improvements. But as the clinical use of the old IR nicorandil demonstrates, there is much to improve in order to enhance the performance and stable delivery of nicorandil.
In that regard, the largest market in Europe — Germany —has a particularly high barrier for entry of new treatments, as it follows a complex process for approving reimbursement for the majority of German patients insured under statutory health insurance plans (Krankenkassen).
Finally, it is often challenging to explain and convince investors of the concept’s validity and its return on investment potential, as most VCs tend to focus on “moonshots” rather than opportunities to meaningfully improve existing medications. What is clear, however, is that the closer we are to marketing approval, the more interest there is from both financial and pharmaceutical partners.
What advice do you have for other biotechs trying to create therapeutics for unmet medical needs?
Litwiniuk: We, of course, laud every team that undertakes the complex, high-risk process of developing new, innovative medications to improve the treatment of the most common and/or most underserved medical conditions plaguing humanity. There is no single concept approach to success here.
In any case, it is critical to have a relentlessly optimistic attitude in view of all the challenges, as there are plenty of reasons to shy away from such a daunting undertaking. What we have noticed as crucial elements among our core team are a broad range of competencies across clinical, regulatory, financial, marketing, and R&D. Thorough understanding of why the need remains unmet despite significant resources being available from big and midsize pharma is important for everyone. Early in the process, it is vital to develop a realistic analysis of both future market size — especially if it is in a sub-segment of the targeted therapeutic area — and anticipated pricing level in future markets.
We started working together as a team during the COVID pandemic and quickly learned that managing collaborative work across broad geographic distances (our team is spread out between Europe and North America) is quite possible without significant loss of information. The best partner is not always close by.
References
- Boden W et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N Engl J Med.2007;356(15):1503-16.
- Maron DJ et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382;1395-407.
- The IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359;1269-75.
About The Experts
 Jed Litwiniuk, MHA, CEO of Auxilius Pharma, is a pharmaceutical and healthcare executive as well as corporate finance professional with over 15 years of experience. Before cofounding Auxilius Pharma, he worked for a range of institutions investing in the healthcare space. His experience includes head of M&A at Lux Med and investment director at both Enterprise Venture Fund and PZU. He is the CEO of Centrum Medyczne Gamma and cofounder of Picket Pharmaceuticals, a NYC-based start-up. He received his executive master of health administration degree at Columbia University Mailman School of Public Health.
Jed Litwiniuk, MHA, CEO of Auxilius Pharma, is a pharmaceutical and healthcare executive as well as corporate finance professional with over 15 years of experience. Before cofounding Auxilius Pharma, he worked for a range of institutions investing in the healthcare space. His experience includes head of M&A at Lux Med and investment director at both Enterprise Venture Fund and PZU. He is the CEO of Centrum Medyczne Gamma and cofounder of Picket Pharmaceuticals, a NYC-based start-up. He received his executive master of health administration degree at Columbia University Mailman School of Public Health.
 Uwe Tigör, MD, CMO, of Auxilius Pharma, has extensive marketing and product launch experience, serving as chief medical officer and medical director in healthcare marketing and communications agencies from IPG, WPP, HAVAS Health to InventivHealth. He received his MD from Humboldt University and did his medical training in the EU and U.S. His cardiovascular research experience includes a research fellowship at Mount Sinai Hospital, NY. He is a master of health administration candidate at Columbia University Mailman School of Public Health.
Uwe Tigör, MD, CMO, of Auxilius Pharma, has extensive marketing and product launch experience, serving as chief medical officer and medical director in healthcare marketing and communications agencies from IPG, WPP, HAVAS Health to InventivHealth. He received his MD from Humboldt University and did his medical training in the EU and U.S. His cardiovascular research experience includes a research fellowship at Mount Sinai Hospital, NY. He is a master of health administration candidate at Columbia University Mailman School of Public Health.
