In Vitro Measurement of [35S]-GTPyS Binding in CHO Cells Expressing the Human 5-HT1B Receptor
![In Vitro Measurement of [35S]-GTPyS Binding in CHO Cells Expressing the Human 5-HT1B Receptor In Vitro Measurement of [35S]-GTPyS Binding in CHO Cells Expressing the Human 5-HT1B Receptor](https://vertassets.blob.core.windows.net/image/85797146/85797146-916f-11d3-9a6a-00a0c9c83afb/159_106-image1.jpg)
Contents
Abstract
Introduction
Methods
Results
Discussion
References
Abstract (Back to Top)
FlashPlate is a 96-well white polystyrene microplate with scintillant-coated wells designed for high-volume, in-plate radiobinding assays. This study investigates the use of FlashPlate as a platform for measuring G-protein activation by means of an assay of [35S]-GTPgS binding in Chinese hamster ovary cells. Results are compared to those obtained using a conventional filtration method.
Introduction (Back to Top)
When an external signal is received by a membrane bound receptor, a series of cellular events takes place which results in the activation of an effector molecule. Many receptors transmit this signal via G-protein activation. This response can be used as a functional measure of receptor-ligand association.
The basic mechanism behind G-protein activation involves the exchange of bound GDP for GTP. Using a radioactive, non-hydrolyzable form of GTP, such as [35S]-GTPgS, G-protein activation can be assessed by measuring the accumulation of bound radioactivity.
We investigated the use of FlashPlate technology (NEN Basic FlashPlate, SMP200) to measure [35S]-GTPgS binding, and briefly compared it to the conventional filtration method. The study examined 5-HT activation of human 5-HT1B receptors expressed in Chinese hamster ovary (CHO) cells.
Receptor Source
A Chinese hamster ovary cell line (ACC098) expressing the human 5-HT1B receptor was used as the receptor source. Prior to [35S]-GTPgS binding assay, the CHO cells were prepared as described by Thomas, et al., 1995, and frozen in aliquots until required.
Filtration Assay
[35S]-GTPgS binding was carried out as described by Watson, et al., 1996. In brief, 108 cells were pre-incubated at 30°C for 30 minutes in HEPES [20 mM], pH 7.4, in the presence of MgCl2 [3 mM], NaCl [100 mM], ascorbate [0.2 mM] and GDP [10 mM] with or without test drugs. The reaction was started by adding 10 µL of [35S]-GTPS [100 pM]. This was followed by a further incubation at 30°C for 30 minutes. The reaction was stopped by rapid filtration, followed by five washes with ice-cold HEPES buffer. Bound radioactivity was determined by liquid scintillation spectrometry. Non-specific binding was determined by 10 µM unlabeled GTggS.
FlashPlate Assay (Membrane Immobilization onto FlashPlate)
CHO cells expressing the h5-HT1B receptor were diluted to a desired membrane protein concentration in 25 mM HEPES buffer containing 2.5 mM CaCl2 and 1 mM MgCl2, at pH 7.4. A suspension of diluted receptor (230 µL) was pipetted into each well of a FlashPlate microplate, and the plates were centrifuged at 800 x g for 10 minutes at 4°C. The supernatant was removed and the plates were left overnight at 4°C. (Later studies have shown that 3 hours is sufficient.) Prior to use, 250 µL of 0.5% BSA in the above HEPES buffer was added to each well and left for 30 minutes at room temperature, in order to block non-specific binding sites.
GTPgS Binding Assay on FlashPlate
This method is essentially similar to the filtration method, with some modifications. The same assay buffer was used for both methods.
- 50 µL of test drug added to appropriate well
- 50 µL of assay buffer (basal) or 50 µL of GTggS (NSB) added to appropriate well
- 50 µL of GDP added to all wells
- 100 µL of buffer added to all wells
The FlashPlate was then incubated for 30 minutes at 30°CC. To start the reaction, 50 µL [35S]-GTPgS (200 pM) was added, and the plates were shaken for 30 seconds. After a further 30 minutes, the reaction was stopped by aspirating the contents of each well. The FlashPlate was then immediately placed on a Packard TopCount Microplate Scintillation and Luminescence Counter to measure bound radioactivity.
Results (Back to Top)
Several experiments were performed to determine the optimum conditions for binding in FlashPlate coated with CHO cell membranes expressing the h5-HT1B receptor. The maximum response produced by 5-HT [10 µM] was achieved when approximately 20 µg of membrane protein was used to coat each well and 10 µM GDP was used in the assay (Figure 1a). This is further supported by s 1b and 1c, which show complete 5-HT concentration response curves. The best signal-to-noise ratio was seen at the above-mentioned protein and GDP concentrations (Figures 1b and 1c, respectively). Figure 1d represents a concentration response curve for GTggS and shows that concentrations in excess of 10 nM are adequate to estimate non-specific binding.
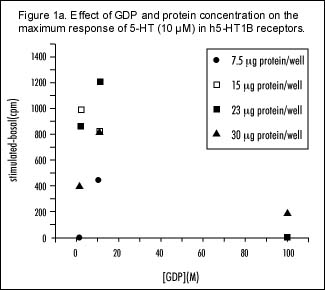
Maximum response to 5-HT (10 µM) was measured on the FlashPlates at varying concentrations of GDP and varying amounts of h5-HT1B receptor protein per well.
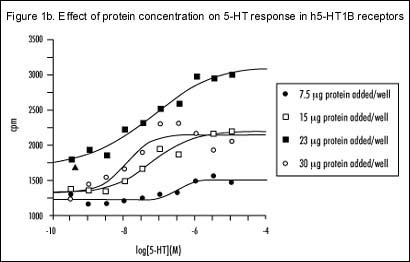
Dose response curves to 5-HT at varying amounts of h5-HT1B receptor protein per well.
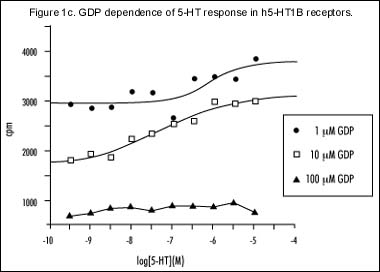
Dependence of dose response curves to 5-HT at varying GDP concentrations.
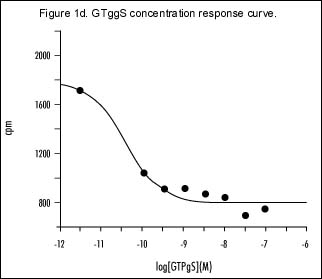
Dose response curves to GTggS in the presence of 10 µM GDP, and at approximately 20 µg per well of h5-HT1B receptor protein added to the FlashPlates.
Intra-assay variation was investigated by measuring the stimulation of [35S]-GTPgS binding produced by 5-HT [10 µM] in 80 of the FlashPlate"s 96 wells. Figure 2a shows the data in terms of absolute counts per minute; Figure 2b shows the same data with each row expressed as a percentage of its individual basal value. Preliminary results on inter-assay variation over three experiments indicate only up to 6% error on mean values (Figure 3a). Further experiments would be required to provide more detail on variability. For comparative studies, concentration response curves to 5-HT, GR127935 and SB-224289 were carried out using FlashPlate and the filtration method (Figures 3a and 3b, respectively). In both assays 5-HT appears as a full agonist, GR127935 acts as a partial agonist and SB-224289 appears as an inverse agonist. The maximum response of 5-HT and GR127935 differs between the two methods (Table 1), but the intrinsic activity of GR127935 is comparable. The maximum response produced by SB-224289 is similar for both assay systems. The comparison between the two methods is summarized in Table 2.
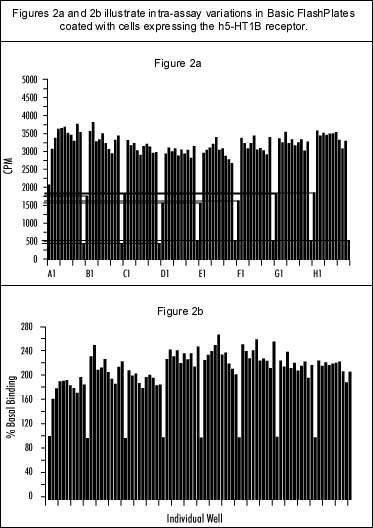
Intra-assay variation of the stimulation of [35S]-GTPgS binding by 5-HT (10 µM) in 80 wells of a 96-well FlashPlate is shown a) plotting in counts per minute, and b) as a percentage of the group"s basal value. Eight groups are shown (one per row of wells on the FlashPlate), starting with a basal in position X1, followed by ten 5-HT stimulations, and finishing with an NSB (10 µM GTggS) determination in position X12.
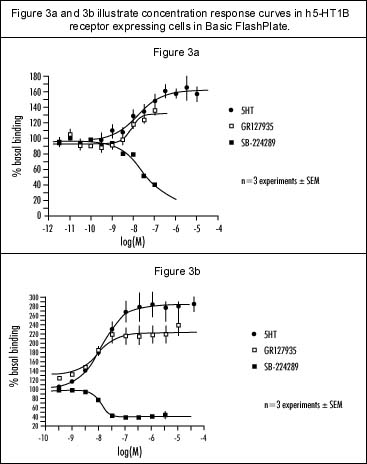
Comparison of dose response curves for 5-HT, GR127935 and SB-224289 were performed using a) FlashPlate methodology, and b) the filtration methodology.

Discussion (Back to Top)
This study's aim was to investigate the efficacy of NEN's Basic FlashPlate as a tool for measuring [35S]-GTPgS binding, using CHO cells expressing the human 5-HT1B receptor as the receptor source. After optimal conditions were assessed, significant stimulation of binding was achieved in the presence of an agonist. The FlashPlate system was also sensitive enough to pick up partial and inverse agonists. (Assessment of optimal conditions will be essential when designing other assays on FlashPlate, for different receptors expressed in different cell lines.)
The FlashPlate-based method agreed closely with the conventional filtration method in terms of potencies and intrinsic activities of the compounds tested, but differences in maximum responses were seen. Intra- and inter-assay variation present in the FlashPlate system may play a role in this, and might be attributable to non-uniform coating of membrane protein to wells. The pipetting procedure adopted, or the volume of residual reaction mixture after aspiration may also be factors. This may not be a major concern if the window of response is adequate for the given application. In cases where intra- and inter-assay variation is a problem, it might be addressed by performing assays in duplicate or greater multiples.
Significantly, total assay time was reduced from 12 hours for the filtration method to 4 hours on FlashPlate. Labor time was also reduced significantly. The FlashPlate method also allows greater scope for automation, which would further reduce labor and increase throughput.
In conclusion, there is great potential for using FlashPlate technology to study [35S]-GTPgS binding in receptor-expressing cell lines.
- Thomas, D.R., et al. (1995) J. Rec. Sign Trans. Res. 15:199
- Watson, J., et al. (1996) Eur. J. Pharmacol. 314:365-372
![In Vitro Measurement of [35S]-GTPyS Binding in CHO Cells Expressing the Human 5-HT1B Receptor](https://vertassets.blob.core.windows.net/sites/logos/drug.png)