From Matrix To Medicine: New Frontiers In Treating Pulmonary Fibrosis And PH
A conversation with Paul Bollyky, Harry Karmouty-Quintana, and Anissa Kalinowski
Halo Biosciences is building a differentiated pipeline based on novel insights into how extracellular matrix (ECM) dysregulation drives disease, focusing on the treatment of inflammatory and fibrotic diseases. The company’s first indication is pulmonary hypertension and interstitial lung disease. In this Q&A, Life Science Connect’s Michelle Raley catches up with leaders to discuss the company’s approach to drug targeting and early drug development. The experts are:
- Paul Bollyky, MD, professor of medicine at Stanford University and scientific cofounder of Halo Biosciences
- Harry Karmouty-Quintana, associate professor, UTHealth Houston, who discovered the hyaluronan-related mechanisms that led to the SATURN trial; currently chair-elect of the ATS Pulmonary Circulation Assembly Program Committee
- Anissa Kalinowski, CEO and cofounder of Halo Biosciences, and a veteran biotech leader with prior roles at Genentech and Roche.
How can extracellular matrix (ECM) modulation alter disease trajectory for pulmonary hypertension and interstitial lung disease (PH-ILD) or other diseases?

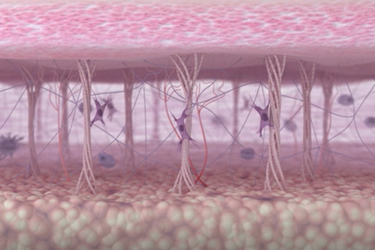
In preclinical models of PH, HA is associated with proliferation of pulmonary artery smooth muscle cells and aberrant vascular remodeling. In humans, elevated HA levels have been reported in several chronic lung diseases. And of specific relevance to our work, increased HA levels also have been reported in autoimmune diseases such as systemic sclerosis linked to connective tissue disease, in interstitial lung disease, and in PH-related lung diseases, including pulmonary fibrosis. Collectively, a growing body of evidence points to a likely pathogenic role for HA in PH and suggests that therapies targeting HA may serve as novel reverse-remodeling treatments in the disease.
In preclinical work by our team and collaborators, we have had an opportunity to study modulation of the ECM with a hyaluronan inhibitor, 4-methylumbelliferone (4-MU). We have seen encouraging results in terms of impact on PH-relevant biomarkers, lung histology, and other measures of disease. And one of the things that is most exciting about the Thorax publication is that we now have early evidence of the potential clinical utility of this ECM modulator from a randomized, placebo-controlled trial.
Your work has shown that inhibiting the synthesis of hyaluronan has the potential to safely downregulate various inflammatory and fibrotic cell-signaling pathways. Can you tell us more about this and how it has potential for treating disease?

One key component of the ECM is hyaluronan, a sugar-like molecule typically known for its beneficial role in the skin and joints. But in lung diseases, we find hyaluronan building up in places where it shouldn’t be, such as small blood vessels and air sacs where oxygen exchange takes place. This abnormal accumulation is harmful because, during inflammation, hyaluronan can break into smaller fragments that promote cell poliferation, altered metabolism, and increased stiffness, hallmarks of fibrosis and pulmonary hypertension.
Our research shows that by preventing the abnormal production and buildup of hyaluronan using a compound called 4-MU, we can halt the progression of lung fibrosis and pulmonary hypertension. This opens the door to a promising new treatment strategy focused on the ECM.
HB-1614 is a proprietary formulation of 4-methylumbelliferone (4-MU). How did you approach formulation development?
Bollyky: Our early clinical studies, including Phase 1 and 2 trials, were conducted using the parent compound, 4-MU. However, these efforts highlighted several pharmacological challenges — most notably, extensive first-pass hepatic metabolism and the relatively high oral doses required to achieve therapeutic effects.
In response, we initiated a formulation program focused on bypassing liver metabolism to improve systemic bioavailability. Over the past year, we’ve concentrated on formulation optimization and preclinical validation to enhance the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of the drug. These efforts are central to the development of our proprietary candidate, HB-1614, and form the foundation of our next phase of clinical investigation.
What development challenges have you encountered in setting up for an IND for the new formulation?

The last year has been dedicated to several foundational efforts. First, we’ve been manufacturing GMP-grade drug substance to support both preclinical and future clinical work. In parallel, we’ve been testing our formulation to better understand and optimize its PK and PD properties. Another major focus has been the design and initiation of our GLP toxicology studies.
One of our key development priorities is leveraging the clinical insights from our SATURN Phase 2a study, particularly given its recent publication, while still working to optimize the drug’s known limitations. Striking that balance is both technically demanding and strategically nuanced. Every adjustment we consider has potential implications not only for clinical performance but also for the drug’s intellectual property profile and eventual commercial positioning.
Another critical piece of the puzzle is how we assemble the right people to do the work. In larger organizations, there are in-house departments with deep functional expertise across pharmacology, manufacturing, regulatory science, toxicology, and more. At Halo, we have to be more agile. We rely heavily on the expertise of our CRO partners, tap into our personal and professional networks, and also do a lot of real-time learning. It’s a dynamic environment that requires strong collaboration and strategic prioritization.
What keeps us going is maintaining a focus on the patients. We're developing a treatment for a chronic, highly symptomatic condition that significantly affects quality of life and mortality. That long-term goal helps anchor us, especially when we’re navigating tough decisions or technical setbacks.
