End-To-End Capabilities

We have the expertise, technologies, and state-of-the-art facilities at ElevateBio BaseCamp® to deliver cell and gene therapies from concept through commercialization, all under a single roof, right from the start.
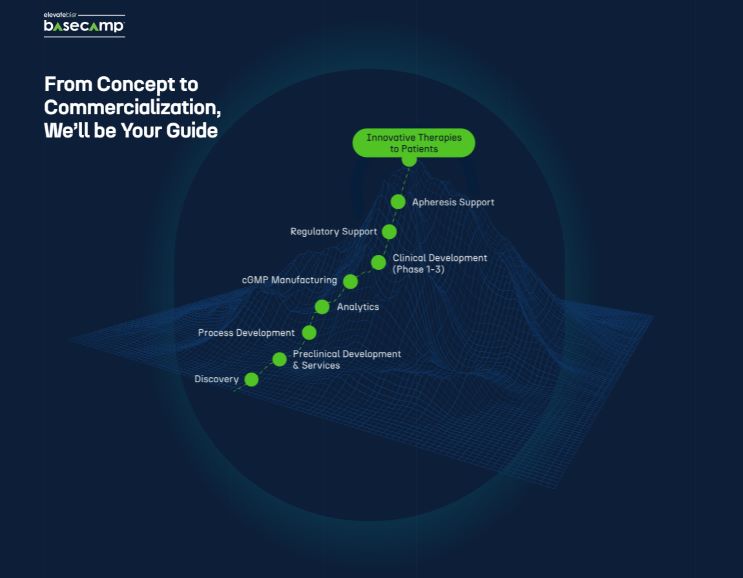
Scaling New Heights, From BaseCamp to Summit
BaseCamp® is our genetic medicine current Good Manufacturing Practice (cGMP) manufacturing and process development business enabling biopharmaceutical partners with end-to-end capabilities.
The purpose-built viral vector and cell therapy centers of excellence provide the tools, expertise, and resources necessary to bring cell and gene therapies and regenerative medicines from concept to commercialization.
We are leading the revolution in cell and gene therapy development, first through our flagship BaseCamp facility in Waltham, Massachusetts and beyond as we expand to new intersections of science, technology, and talent.
Cell Therapies
We provide a full-spectrum solution for cell therapy manufacturing with tools and expertise in place to support across process development, analytical development, CMC regulatory, manufacturing and more.
Viral Vectors
We offer an unmatched array of viral vector services to support across all stages of development, including end-to-end process development and manufacturing for lentiviral and adeno-associated virus (AAV) vectors.
- cGMP Manufacturing And Automation - Our cGMP manufacturing facility is equipped for the full product lifecycle. Click here to learn more.
- Process Development - In cell and gene therapies, the process is the product. We have deep expertise developing and scaling processes for cell therapies and viral vectors, including lentiviral vectors and AAV. Click here to learn more.
- Analytical Services And Quality - Our robust analytical capabilities support development processes and ensure our products are well characterized, safe and meet regulatory guidelines. Click here to learn more.
- Enabling Services - Our team of experts can help you navigate the complex manufacturing and development lifecycle for cell and gene therapies from bench to bedside. Click here to learn more.
Our Customizable LentiPeak™ Platform
Our proprietary lentiviral vector (LVV) platform can be completely customized to meet your specific development needs. LentiPeak™, our suspension-based, scalable lentiviral vector production platform, enables efficient transition for cell and gene therapies from preclinical stage through clinical development and commercialization with accelerated timelines and reduced manufacturing costs. Click here to learn more.
Partner with ElevateBio®
Wherever you are in your cell and gene therapy product lifecycle, we can strengthen and accelerate the development of your transformative therapies with our enabling technologies unmatched manufacturing capabilities.