mRNA: The Fourth Pillar Of Pharmaceutical Innovation And Intervention
By Deborah Day Barbara, the Alliance for mRNA Medicines

As I ascended the weathered steps of the Parthenon last summer I was struck by the strength and endurance of the Parthenon’s pillars that are unyielding to the elements and to time. Like the ancient columns, the pharmaceutical pillars are foundational innovations that support the advancement of the pharmaceutical sciences. Reflecting on pharmaceutical history, one can discern three pharmaceutical pillars that have shaped the biotech and pharmaceutical industry: small molecules, biologics, and cell and gene therapies. With the rise of mRNA medicines, it is becoming increasingly evident that we are witnessing the genesis of a fourth pillar. I contend that the formation of this fourth pillar will deliver much needed innovative medicines to patients, be a driving force of bio/pharma industry growth, and pave the way for future pillars.
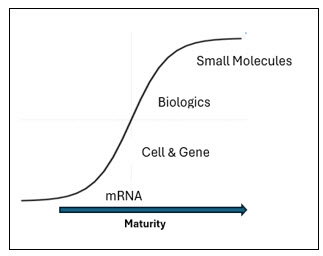
Pharmaceutical Pillars Defined
Pharmaceutical pillars are discrete categories of innovative medicines comprising a combination of similar attributes including: 1) molecule composition, 2) the category of molecules/cells targeted, 3) a similar manufacturing process, and 4) the breadth of indications that they can potentially address.
For example, consider synthetic small molecules (including TIDES) that are simple chemical structures designed and manufactured by medicinal chemists via organic chemistry. These drugs are often delivered orally and most commonly target specific receptors regulating biological processes and treating a wide range of indications in neurology, ophthalmology, and oncology, among many others. Synthetic small molecules are the most mature of the pharmaceutical pillars.
In contrast, traditional biologics (mAbs, enzyme/proteins) are more complex and difficult to manufacture. These products are delivered intravenously and can be more specifically targeted, potentially resulting in improved safety/efficacy when compared to small molecules.
Pillars Work Together To Support The Same Goal
Though each pillar stands on its own, increasingly we are seeing collaboration among the pharmaceutical pillars — particularly as we explore combination therapies designed to increase drug efficacy.
We can see this collaboration in a variety of areas, but most notable is the emergence of immunotherapy and mRNA neoantigen vaccine combination therapies to treat cancer. For example, Moderna Therapeutics and Merck & Company are collaborating on a combination medicinal approach that brings together the biologics pillar and the mRNA pillar. Moderna’s mRNA-4157/V940 — an investigational individualized neo-antigen mRNA cancer vaccine in combination with Merck’s anti PD-1 therapy, KEYTRUDA(R) — is being used to treat high-risk melanoma patients following resection. Early-stage clinical results proved to be very promising, and Merck is currently recruiting patients for a Phase 3 clinical trial.1
Pillars Represent A Continuum Of Foundational Medicinal Innovation
In addition to an increase in “cross-pillar” combination therapies, I think it’s interesting to note how our industry’s therapeutic goals also have evolved with the construction of each pillar. As the pharmaceutical industry has progressed from the most mature pharmaceutical pillar (small molecules) to the most nascent pharmaceutical pillar (mRNA therapies), our medicines have advanced from being generalized therapies to more personalized/targeted therapies that place the patient in the center of the manufacturing process. Small molecules and biologicals target receptors and biological pathways in a general manner. In contrast, cell and gene therapies and mRNA (non-prophylactic) vaccines and therapies often leverage the patient as the bioreactor, targeting diseased cells or deploying the patient’s immune system, enabling the patient to heal themselves.
Defining & Strengthening The mRNA Pharmaceutical Pillar
The regulatory approvals of the first mRNA-based vaccines (Comirnaty and Spikevax) have positioned mRNA to rise as the fourth pillar of pharmaceutical intervention and innovation. Since approval of Comirnaty and Spikevax, the success of mRNA technology has attracted billions of dollars in private investment to broaden mRNA’s prophylactic and therapeutic applications.2 In turn, we’ve seen Big Pharma companies revive their nucleic acid therapy programs; new biotech companies have formed; and medicinal pipelines have expanded from a few mRNA molecules to greater than 900 molecules in preclinical and clinical development.3 The recent news from Capstan Therapeutics regarding its “up round” of $175 million Series B following its $63 million seed and September 2022 $102 million Series A is a testament to the private equity market’s enthusiasm for the promise of mRNA beyond mRNA vaccines.4Unlike the other pharmaceutical pillars that are made from very simple chemicals or significantly more complex biological compositions, mRNA vaccines and therapeutics comprise nucleotide triphosphates (NTPs) and base modified NTPs that are stitched together in the lab with enzymes to make synthetic mRNA. The innovation of nucleoside base modification was the foundational discovery for which the 2023 Nobel prize in medicine was awarded to Katalin Kariko and Drew Weissman. The innovation in nucleoside chemistry fundamentally changes our understanding of how mRNA interacts with the immune system.
Likewise, the manufacturing process for mRNA — in vitro transcription — is a significantly different manufacturing process than that of biologicals and cell and gene therapies, all of which are reliant on large bioreactors, cell lines/patient cells, and significant media consumption. Cell-free manufacturing provides the advantages of simplicity, flexibility, and cost-effectiveness over cell-based manufacturing processes, positioning mRNA as the ideal “plug and play” platform that reduces vaccine and drug development timelines, speeding critical medicines to market in a fraction of the time.5
mRNA offers for the first time the ability to harness the patient’s own cellular machinery to express a gene in vivo. This gene may be a simple antigen to elicit an immune response like the COVID-19 mRNA vaccines, or it could encode complex sequences such as those of a therapeutic monoclonal antibody. This molecule flexibility allows mRNA to potentially treat a wide range of diseases as a prophylactic vaccine, a personalized cancer vaccine, a protein replacement therapy, a therapeutic protein, and/or as a critical technology enabling gene-editing (e.g., CRISPR). These molecules have the potential power to protect the world from malaria, HIV, and other deadly pathogens, provide a cure for cancer, treat sickle cell anemia (like newly approved CASGEVY), and other diseases/conditions where there is a significant unmet need.
While the mRNA field is excited about the medicinal possibilities, there are several critical technical challenges we must solve to strengthen and increase the height of the mRNA pillar, including the stability and deliverability of our mRNA vaccines and therapies.
Improving mRNA medicines’ ability to be stored outside of freezers and targeting specific organs has the potential to greatly expand the medicinal reach of mRNA. For example, researchers are exploring new manufacturing approaches that include lyophilization of the drug product so that vaccines may be safely shipped around the globe without refrigeration/specific handling restrictions. MIT scientists are striving to improve the in vivo stability of mRNA through creative molecule design and have recently published a new Poly A tail design concept that, in rodents, has increased in vivo protein expression from under seven days to up to 14 days, extending the therapeutic utility of mRNA.6
Another area of intensive innovation is organ/tissue-specific mRNA delivery to ensure mRNA drug products reach the intended organ/tissue. Current delivery of mRNA is predominantly focused on ionizable lipid nanoparticles that deliver mRNA to the liver like those used in the COVID-19 vaccine. Researchers are developing non-invasive approaches that deliver mRNA directly to the lungs, heart, spleen, pancreas, and brain. For example, scientists at Carnegie Mellon University are seeking new mRNA delivery approaches via novel formulations7and routes of administration8 to successfully deliver mRNA to disease-specific organs.
mRNA Pillar Is Also Strengthened Through Advocacy
As with the other pillars, the mRNA therapeutic innovators and other key stakeholders have taken the important step to organize the community by establishing a global advocacy organization, the Alliance for mRNA Medicine (AMM). The AMM launched officially on Oct. 31, 2023, and was introduced at the 12th International mRNA Health Conference in Berlin. The organization has already recruited more than 50 leading companies and institutions in the sector. AMM will coordinate and lead industry and institutional efforts worldwide to work with regulators, policy makers, standards development organizations, and patient groups to advance scientific knowledge and create the safest and most efficient path for the adoption of new mRNA medicines. AMM is already actively engaged in efforts to promote international harmonization of evolving regulations to guide therapeutic and vaccine development in the sector and to help shape guidance on platform designations for technologies key to mRNA therapeutic development.
mRNA COVID-19 vaccines were only the beginning. Science is surging forward, expanding mRNA pipelines, and addressing important technical challenges. A new pharmaceutical pillar of safe, efficacious, cost-effective, patient-centered medicines that can stand the test of time like the pillars of the Parthenon has arrived.
References
- Merck and Moderna Initiate Phase 3 Study Evaluating V940 (mRNA-4157) in Combination with KEYTRUDA (pembrolizumab) for Adjuvant Treatment of Patients with Resected High-Risk (Stage IIB-IV) Melanoma (modernatx.com)
- mRNA COVID vaccines saved lives and won a Nobel — what’s next for the technology? (nature.com)
- https://www.linkedin.com/posts/mike-cao-b9b267171_mrna-rna-rnatherapeutics-activity-7174358847761829888-uvEm?utm_source=share&utm_medium=member_desktop
- https://endpts.com/cell-therapy-withouT cells-capstan-raises-175m-to-test-in-vivo-car-t/
- https://www.linkedin.com/posts/mike-cao-b9b267171_mrna-rna-rnatherapeutics-activity-7174358847761829888-uvEm?utm_source=share&utm_medium=member_desktop
- Branched chemically modified poly(A) tails enhance the translation capacity of mRNA | Nature Biotechnology
- The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs - PubMed (nih.gov)
- https://pubmed.ncbi.nlm.nih.gov/36706177/
About The Author:
 Deborah “Deb” Day Barbara, with the Alliance for mRNA Medicines, is the current Chairperson for AMM’s sister organization, the Foundation for mRNA Medicine. She has held many life sciences industry roles as company builder, advisor, investor, and industry advocate. Her career has largely focused on developing and commercializing innovative new products and services to enable the regulated markets with several companies and institutions, including Amersham Life Sciences, The Johns Hopkins University, Gene Logic, Strategic Diagnostics, Life Technologies, Thermo Fisher Scientific, and Maravai Life Sciences — all while contributing significant efforts to the support of associations and not-for-profit entities. Currently, Day Barbara continues to guide small and midsize companies toward growth and identify and promote technologies that are transforming the life sciences industry. She is also passionate about spending time mentoring and coaching the next generation of life sciences industry leaders.
Deborah “Deb” Day Barbara, with the Alliance for mRNA Medicines, is the current Chairperson for AMM’s sister organization, the Foundation for mRNA Medicine. She has held many life sciences industry roles as company builder, advisor, investor, and industry advocate. Her career has largely focused on developing and commercializing innovative new products and services to enable the regulated markets with several companies and institutions, including Amersham Life Sciences, The Johns Hopkins University, Gene Logic, Strategic Diagnostics, Life Technologies, Thermo Fisher Scientific, and Maravai Life Sciences — all while contributing significant efforts to the support of associations and not-for-profit entities. Currently, Day Barbara continues to guide small and midsize companies toward growth and identify and promote technologies that are transforming the life sciences industry. She is also passionate about spending time mentoring and coaching the next generation of life sciences industry leaders.
