Encouraging Fibromyalgia Study Results Now Available At ClinicalTrials.gov
San Diego, CA /PRNewswire/ - AVACEN Medical announced today that ClinicalTrials.gov, a service of the U.S. National Institutes of Health, has published the results of the Company's Fibromyalgia clinical trials. ClinicalTrials.gov is a web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions.
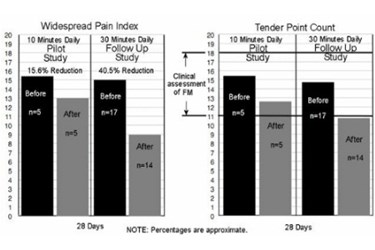
The study was conducted by The University of California, San Diegoand the Department of Veterans Affairs. The study results, also published in the Society for Neuroscience (SfN) annual meeting and poster program, indicated the 28 day AVACEN follow-up study exhibited a statistically significant reduction of over 40% in the widespread pain index and a decrease in tender point count to a level below the value typically used for the clinical assessment of fibromyalgia.
These results were derived from a treatment process that uses the palm to noninvasively transfer heat into the circulatory system while the body is at normothermia (approximately 98.6 degrees F). The Company refers to this process as the AVACEN Treatment Method.
According to AVACEN Medical CEO and inventor, Thomas Muehlbauer, "These results can significantly improve the quality of life for fibromyalgia sufferers worldwide. We have submitted this data to the FDA and are eager to receive approval to market our device for relief of widespread pain associated with fibromyalgia. This disease affects approximately 10 million people in the U.S. and an estimated 3-6% of the global population."
Muehlbauer added, "Our patented AVACEN Treatment Method is an entirely new concept in chronic pain treatment through whole-body muscular relaxation. This is accomplished by bathing the skeletal muscles with warm oxygenated and nutritious blood. Our FDA-Cleared AVACEN 100 uses the AVACEN Treatment Method."
"The key is the continued infusion of heat into the circulatory system through the palm when the body is at normothermia, approximately 98.6 degrees F. The heat acts as a catalyst to reduce the thickness of the blood. The result is the body must dissipate the unwanted heat by pumping the warmed and thinner blood through the skeletal muscles to reach the heat exchange capillary network where it can be cooled by the ambient air. It should be noted that the level of heat is precisely controlled so that it cannot induce heatstroke. Actually, the level of infused heat normally doesn't even engage the sweat glands."
"Understanding our use of the palm as a single point of treatment is challenging for most. Essentially, the heart pumps the warmed and thinner blood originating from our treatment of the palm to all organ and muscle tissues. Therefore, wherever blood flows, enhanced oxygen delivery and nutrition can occur. The positive effects on the progression of aging, longevity, and quality of life could be enormous!"
Current first line therapies for the multi-billion dollar fibromyalgia pain treatment market are Cymbalta (duloxetine), Lyrica (pregabalin), Savella (Milnacipran), and Elavil (Amitriptyline); but only a few patients obtain any sustainable relief of pain. The potential adverse effects of these drugs often outweigh the benefit for most patients. There are currently no known FDA approved or cleared medical devices that treat the widespread pain associated with fibromyalgia. The AVACEN 100 has a record of approximately 500,000 treatments without a single reported negative side effect.
About AVACEN Medical: Over the last two years, AVACEN Medical has been awarded 3 U.S. and 5 International patents for its unique apparatus and methods that support its innovative medical process called the AVACEN Treatment Method. The company is dedicated to the innovation and design of safe, easy to use, noninvasive drug-free alternatives to treat chronic pain. Founded in 2009, AVACEN is headquartered in San Diego. Contact: Ryan Jeffcoat at (888) 4-AVACEN x 711 or Email.
*The AVACEN 100 is pending FDA approval for widespread pain related to fibromyalgia. It is FDA-Cleared as a Class II heat therapy system indicated for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis, muscle spasms, minor strains and sprains; muscular relaxation. It is not available for sale for any non-cleared indication mentioned in this document.
Source: AVACEN Medical
Copyright 2015 PR Newswire. All Rights Reserved