Avigen completes new manufacturing facility
Second site focuses on gene therapy products
Avigen Inc. (Alameda, CA) has completed construction of its second manufacturing facility. The 10,300-square-foot plant, located adjacent to Avigen's headquarters, incorporates robotics technology and is designed to produce commercial-scale quantities of adeno-associated virus (AAV) vectors. An additional 12,000-sq-ft facility for process development is scheduled for completion in November.
Avigen is entering the second year of a clinical trial of Coagulin-B, its gene therapy product for hemophilia B. A similar product for correcting the Factor VIII deficiency that is associated with hemophilia A is in pre-clinical testing.
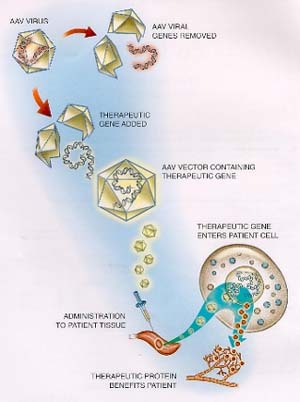
Avigen uses non-pathogenic viruses to deliver therapeutic genes to target tissues.
AAV vectors are based on simple, common viruses with which nearly every human being has already been infected. The simple AAV contains only two genes. One is the "Rep" gene, which codes for proteins involved in DNA replication. The other is the "CAP" gene, which, by differential splicing, codes for three proteins that makes up the protein coat of the virus.
"This second facility provides us the infrastructure to produce AAV vectors for two independent clinical trials, such as those for Factor VIII and late phase Factor IX," noted Avigen president and CEO John Monahan. "Additionally, we believe this is the first manufacturing facility capable of producing commercial-scale quantities of AAV. Supported by our progress in clinical trials and our expanding AAV intellectual property position, Avigen remains committed to being the first company with a gene therapy product for patients with hemophilia."
The new facility is designed to meet the FDA's Good Manufacturing Practices (GMP) requirements and incorporates Avigen's need for full-scale commercial production of AAV vectors. The company's patented processes enable the production of AAV vectors without using a helper virus, such as infectious adenovirus, that may be pathogenic. Avigen has also upgraded the manufacturing process to incorporate larger-scale, column-based purification methodology.
In the current dose escalation trial, the first patient in the high dose group has now been injected. Six patients have received intramuscular injections at low and medium doses according to the protocol. All patients tested to date have demonstrated successful gene expression of Factor IX in muscle tissue, and the treatment has been well tolerated. Avigen has also announced a proposed clinical trial of gene therapy utilizing its AAV vector to deliver genes for Factor IX via the liver.
For more information: Avigen Inc., 1201 Harbor Bay Parkway, Suite 1000, Alameda, CA 94502. Tel: 510-748-7150. Fax: 510-748-7155.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online
